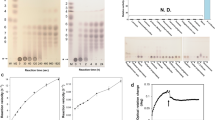
Abstract
Osmoregulated periplasmic glucans are a family of oligosaccharides found in the periplasm of Gram negative bacteria. Mutants devoid of OPGs show strong reduction or absence of virulence on their hosts and display pleiotropic phenotype. Glucose is the sole constituent sugar and OPG level increases as the osmolarity of the medium decreases. OPG synthesis is regulated both at the transcriptional and at the enzymatic level. Data presented in this article indicate that in addition, OPG synthesis requires constant synthesis of protein indicating rapid turnover of one of the two proteins catalyzing glucose backbone of OPGs.



Similar content being viewed by others
References
Arellano-Reynoso B, Lapaque N, Salcedo S et al (2005) Cyclic beta-1, 2-glucan is a Brucella virulence factor required for intracellular survival. Nat Immunol 6:618–625
Bhagwat AA, Jun W, Lui L et al (2009) Osmoregulated periplasmic glucans of Salmonella enterica serovar Typhimurium are required for optimal virulence in mice. Microbiology 155:229–237
Bohin JP (2000) Osmoregulated periplasmic glucans in Proteobacteria—a minireview. FEMS Microbiol Lett 186:11–19
Bohin JP, Lacroix JM (2006) The periplasm. Washington DC, ASM Press
Bouchart F, Delangle A, Lemoine J et al (2007) Proteomic analysis of a non-virulent mutant of the phytopathogenic bacterium Erwinia chrysanthemi deficient in osmoregulated periplasmic glucans: change in protein expression is not restricted to the envelope, but affects general metabolism. Microbiology 153:760–767
Byers DM, Gong H (2007) Acyl carrier protein: structure-function relationships in a conserved multifunctional protein family. Biochem Cell Biol 85:649–662
Cogez V, Talaga P, Lemoine J et al (2001) Osmoregulated periplasmic glucans of Erwinia chrysanthemi. J Bacteriol 183:3127–3133
Dartigalongue C, Missiakas D, Raina S (2001) Characterization of the Escherichia coli σE regulon. J Biol Chem 276:20866–20875
Debarbieux L, Bohin A, Bohin JP (1997) Topological analysis of the membrane-bound glucosyltransferase, MdoH, required for osmoregulated periplasmic glucan synthesis in Escherichia coli. J Bacteriol 179:6692–6698
Hanoulle X, Rollet E, Clantin B et al (2004) Structural analysis of Escherichia coli OpgG, a protein required for the biosynthesis of osmoregulated periplasmic glucans. J Mol Biol 342:195–205
Lacroix JM, Loubens I, Tempête M et al (1991) The mdoA locus of Escherichia coli consists of an operon under osmotic control. Mol Microbiol 5:1745–1753
Lacroix JM, Tempête M, Menichi B et al (1989) Molecular cloning and expression of a locus (mdoA) implicated in the biosynthesis of membrane-derived oligosaccharides in Escherichia coli. Mol Microbiol 3:1173–1182
Lequette Y, Rollet E, Delangle A et al (2007) Linear osmoregulated periplasmic glucans are encoded by the opgGH locus of Pseudomonas aeruginosa. Microbiology 153:3255–3263
Mahajan-Miklos S, Tan MW, Rahme LG et al (1999) Molecular mechanisms of bacterial virulence elucidated using a Pseudomonas aeruginosa-Caenorhabditis elegans pathogenesis model. Cell 96:47–56
Mills D, Mukhopadhyay P (1990) Pseudomonas: biotransformation, pathogenesis, and evolving biotechnology. ASM Press, Washington DC
Neidhardt FC, Umbarger HE (1996) Escherichia coli and Salmonella typhimurium Cellular and molecular biology, 2nd edn. ASM Press, Washington DC
Page F, Altabe S, Hugouvieux-Cotte-Pattat N et al (2001) Osmoregulated periplasmic glucan synthesis is required for Erwinia chrysanthemi pathogenicity. J Bacteriol 183:3134–3141
Rhodius VA, Suh WC, Nonaka G et al (2006) Conserved and variable functions of the σE stress response in related genomes. PLoS Biol 4:e2
Rumley MK, Therisod H, Weissborn AC et al (1992) Mechanisms of regulation of the biosynthesis of membrane-derived oligosaccharides in Escherichia coli. J Biol Chem 267:11806–11810
Schneider JE, Reinhold V, Rumley MK et al (1979) Structural studies of the membrane-derived oligosaccharides of Escherichia coli. J Biol Chem 254:10135–10138
Talaga P, Fournet B, Bohin JP (1994) Periplasmic glucans of Pseudomonas syringae pv. syringae. J Bacteriol 176:6538–6544
Thérisod H, Weissborn AC, Kennedy EP (1986) An essential function for acyl carrier protein in the biosynthesis of membrane-derived oligosaccharides of Escherichia coli. Proc Natl Acad Sci USA 83:7236–7240
Vinopal RT, Hillman JD, Schulman H et al (1975) New phosphoglucose isomerase mutants of Escherichia coli. J Bacteriol 122:1172–1174
Weissborn AC, Rumley MK, Kennedy EP (1992) Isolation and characterization of Escherichia coli mutants blocked in production of membrane-derived oligosaccharides. J Bacteriol 174:4856–4859
Acknowledgments
This study was supported by grants from the Centre National de la Recherche Scientifique.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lacroix, JM., Bohin, JP. Osmoregulated Periplasmic Glucan Polymerization Requires Constant Protein Synthesis in Escherichia coli . Curr Microbiol 61, 396–400 (2010). https://doi.org/10.1007/s00284-010-9625-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-010-9625-2