Abstract
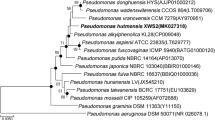
A Gram-negative, mobile, rod-shaped, non-spore-forming bacterium (strain 14-3T) was isolated from a temporary pond in Antarctica. On the basis of 16S rRNA gene sequence similarity, strain 14-3T was shown to belong to the genus Pseudomonas sensu stricto. Physiological and biochemical tests supported the phylogenetic affiliation. Strain 14-3T is closely related to Pseudomonas veronii DSM 11331T, sharing 99.7% sequence similarity. DNA–DNA hybridization experiments between the two strains showed only moderate reassociation similarity (35.1%). Tests for arginine dihydrolase and nitrate reduction were positive, while those for denitrification, indol production, glucose acidification, urease, ß-galactosidase, esculin, caseine and gelatin hydrolysis were negative. Growth of this bacterium occurred in a range from 4 to 37°C but not at 42°C. It accumulated poly(3-hydroxybutyrate) when grown on sodium octanoate medium. Strain 14-3T therefore represents the type strain of a new species, for which the name Pseudomonas extremaustralis sp. nov. is proposed. The type strain 14-3T has been deposited as DSM 17835T and as CIP 109839T.

Similar content being viewed by others
References
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl Acids Res 25:3389–3402
Anzai Y, Kim H, Park JY, Wakabayashi H, Oyaizu H (2000) Phylogenetic affiliation of the pseudomonads based on 16S rRNA sequence. Int J Syst Bacteriol 50:1563–1589
Ayub ND, Pettinari MJ, Ruiz JA, López NI (2004) A polyhydroxybutyrate-producing Pseudomonas sp. isolated from Antarctic environments with high stress resistance. Curr Microbiol 49:170–174
Ayub ND, Pettinari MJ, Méndez BS, López NI (2006) Impaired polyhydroxybutyrate biosynthesis from glucose in Pseudomonas sp. 14-3 is due to a defective beta-ketothiolase gene. FEMS Microbiol Lett 264:125–131
Ayub ND, Pettinari MJ, Méndez BS, López NI (2007) The polyhydroxyalkanoate genes of a stress resistant Antarctic Pseudomonas are situated within a genomic island. Plasmid 58:240–248
Ayub ND, Tribelli PM, López NI (2009) Polyhydroxyalkanoates are essential for maintenance of redox state in the Antarctic bacterium Pseudomonas sp. 14-3 during low-temperature adaptation. Extremophiles 13:59–66
Behrendt U, Ulrich A, Schumann P (2003) Fluorescent pseudomonads associated with the phyllosphere of grasses; Pseudomonas trivialis sp. nov., Pseudomonas poae sp. nov. and Pseudomonas congelans sp. nov. Int J Syst Evol Microbiol 53:1461–1469
Bozal N, Montes MJ, Mercadé E (2007) Pseudomonas guineae sp. nov., a novel psychrotolerant bacterium from an Antarctic environment. Int J Syst Evol Microbiol 57:2609–2612
Braunegg G, Sonnleitner B, Lafferty RM (1978) A rapid gas chromatographic method for the determination of poly-ß-hydroxybutyric acid in microbial biomass. Eur J Appl Microbiol Biotechnol 6:29–37
Bruni V, Gugliandolo C, Maugeri T, Allegra A (1999) Psychrotrophic bacteria from a coastal station in the Ross Sea (Terra Nova Bay, Antarctica). New Microbiol 22:357–363
Cashion P, Hodler-Franklin MA, McCully J, Franklin M (1977) A rapid method for base ratio determination of bacterial DNA. Anal Biochem 81:461–466
De Ley J, Cattoir H, Reynaerts A (1970) The quantitative measurement of DNA hybridization from renaturation rates. Eur J Biochem 12:133–142
de Smet MJ, Eggink G, Witholt B, Kingma J, Wynberg H (1983) Characterization of intracellular inclusions formed by Pseudomonas oleovorans during growth on octane. J Bacteriol 154:870–878
Diard S, Carlier JP, Ageron E, Grimont PAD, Langlois V, Guerin P, Bouvet OMM (2002) Accumulation of Poly(3-hydroxybutyrate) from octanoate in different Pseudomonas belonging to the rRNA homology group I. Syst Appl Microbiol 25:183–188
Elomari M, Coroler L, Hoste B, Gillis M, Izard D, Leclerc H (1996) DNA relatedness among Pseudomonas strains isolated from natural mineral waters and proposal of Pseudomonas veronii sp. nov. Int J Syst Bacteriol 46:1138–1144
Escara JF, Hutton JR (1980) Thermal stability and renaturation of DNA in dimethyl sulfoxide solutions: acceleration of the renaturation rate. Biopolymers 19:1315–1327
Huss VAR, Festl H, Schleifer KH (1983) Studies on the spectrophotometric determination of DNA hybridization from renaturation rates. Syst Appl Microbiol 4:184–192
Ivanova EP, Gorshkova NM, Sawabe T, Hayashi K, Kalinovskaya NI, Lysenko AM, Zhukova NV, Nicolau DV, Kuznetsova TA, Mikhailov VV, Christen R (2002) Pseudomonas extremorientalis sp. nov., isolated from drinking water reservoir. Int J Syst Evol Microbiol 52:2113–2120
Kämpfer P, Kroppenstedt RM (1996) Numerical analysis of fatty acid patterns of coryneform bacteria and related taxa. Can J Microbiol 42:989–1005
Kessler B, Palleroni NJ (2000) Taxonomic implications of synthesis of poly-ß-hydroxybutyrate and other poly-ß-hydroxyalkanoates by aerobic pseudomonads. Int J Syst Evol Microbiol 50:711–713
King EO, Ward MK, Raney DE (1954) Two simple media for the demonstration of pyocyanin and fluorescein. J Lab Clin Med 44:301–307
Kriss AE, Mitskevich IN, Rozanova EP, Osnitskaia LK (1976) Microbiological studies of the Wanda Lake (Antarctica). Mikrobiologiya 45:1075–1081
Kumar S, Tamura K, Nei M (2004) MEGA3: integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief Bioinform 5:150–163
Maugeri TL, Gugliandolo C, Bruni V (1996) Heterotrophic bacteria in the Ross Sea (Terra Nova Bay, Antarctica). New Microbiol 19:67–76
Moore ERB, Mau M, Arnscheidt A, Bottger EC, Hutson RA, Collins MD, Van De Peer Y, De Wachter R, Timmis KN (1996) The determination and comparison of the 16S rRNA gene sequences of species of the genus Pseudomonas (sensu stricto) and estimation of the natural intrageneric relationships. Syst Appl Microbiol 19:478–492
Migula W (1894) Über ein neues System der Bakterien. Arb Bakteriol Inst Karlsruhe 1:235–238
Miller LT (1982) A single derivatization method for bacterial fatty acid methyl esters including hydroxy acids. J Clin Microbiol 16:584–586
Ostle A, Holt JG (1982) Nile Blue A as a fluorescent stain for poly-hydroxybutyrate. Appl Environ Microbiol 44:238–241
Rainey FA, Stackebrandt E (1993) 16S rDNA analysis reveals phylogenetic diversity among the polysaccharolytic clostridia. FEMS Microbiol Lett 113:125–128
Reddy GSN, Matsumoto GI, Schumann P, Stackebrandt E, Shivaji S (2004) Psychrophilic pseudomonads from Antarctica: Pseudomonas antarctica sp. nov., Pseudomonas meridiana sp. nov. and Pseudomonas proteolytica sp. nov. Int J Syst Evol Microbiol 54:713–719
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Shivaji S, Vijama Bhanu N, Aggarwal RK (1989) Isolation and identification of Pseudomonas spp. from Schirmacher Oasis, Antarctica. Appl Environ Microbiol 55:767–770
Timm A, Steinbüchel A (1990) Formation of polyesters consisting of medium-chain-length 3-hydroxyalkanoic acids from gluconate by Pseudomonas aeruginosa and other fluorescent pseudomonads. Appl Environ Microbiol 56:3360–3367
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Vogel HJ, Bonner BN (1956) Acetyl-ornithinase of Escherichia coli: partial purification and some properties. J Biol Chem 218:97–106
Acknowledgments
We thank Dr. Rubén Quintana for collecting Antarctic samples, Sandra Kohaus for technical assistance and Frank Reinecke for help during phylogenetic tree analysis. This work was supported by grants from UBA and CONICET. M.J.P., N.I.L. and B.S.M. are career investigators from CONICET. P.M.T. has a graduate student fellowship from CONICET.
Author information
Authors and Affiliations
Corresponding author
Additional information
The GenBank/EMBL/DDBJ accession number for the 16S rRNA gene sequence of strain 14-3T is AJ583501.
Rights and permissions
About this article
Cite this article
López, N.I., Pettinari, M.J., Stackebrandt, E. et al. Pseudomonas extremaustralis sp. nov., a Poly(3-hydroxybutyrate) Producer Isolated from an Antarctic Environment. Curr Microbiol 59, 514–519 (2009). https://doi.org/10.1007/s00284-009-9469-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-009-9469-9