Abstract
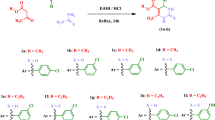
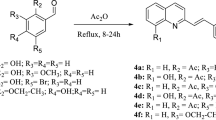
Current drugs for treating leishmaniasis are still associated with significant toxicity and failure rates. Thus, new effective and less toxic antileishmanial agents are still in need. Herein, we tested a series of sulfonamide 4-methoxychalcone derivatives against L. amazonensis promastigote and amastigote forms to identify its antileishmanial profile against this species compared to L. braziliensis. In addition, we used molecular modeling tools to determine stereoelectronic features that may lead to the antileishmanial profile. Interestingly, all tested compounds were able to affect L. amazonensis promastigote form in a concentration-dependent manner and with low cytotoxicity, except for derivative 3g. However, our results showed that compound 3f (para-Cl) presents the best profile against both L. amazonensis forms (promastigote and amastigote), differently from that observed for L. braziliensis, when compound 3i was the most active. Structure–activity relationship (SAR) analysis of these derivatives pointed molecular volume, HOMO density, and conformational aspects as important characteristics for parasitic profile. Overall, sulfonamide 4-methoxychalcone derivatives may be pointed out not only as lead compounds for treating leishmaniasis (i.e., 3f) but also as experimental tools presenting parasite-selectivity (i.e., 3i).



Similar content being viewed by others
References
Ali A (2002) Leishmaniasis and HIV/AIDS co-infections: review of common features and management experiences. Ethiop Med J 40:37–49
Andrighetti-Fröhner CR, Oliveira KN, Gaspar-Silva D, Pacheco LK, Joussef AC, Steindel M, Simões CMO, Souza AMT, Magalhães UO, Afonso IF, Rodrigues CR, Nunes RJ, Castro HC (2009) Synthesis, biological evaluation and SAR of sulfonamide 4-methoxychalcone derivatives with potential antileishmanial activity. Eur J Med Chem 44:755–763
Balanco JMF, Pral EMF, Silva S, Bijovsky AT, Mortara RA, Alfieri SC (1998) Axenic cultivation and partial characterization of Leishmania braziliensis amastigote-like stages. Parasitology 116:103–113
Bhattacharya G, Herman J, Delfin D, Salem MM, Barszcz T, Mollet M, Riccio G, Brun R, Werbovetz KA (2004) Synthesis and antitubulin activity of N-1- and N-4-substituted 3,5-dinitro sulfanilamides against African trypanosomes and Leishmania. J Med Chem 47:1823–1832
Boeck P, Falcão CAB, Leal PC, Yunes RA, Cechinel Filho V, Torres-Santos VEC, Rossi-Bergmann B (2006) Synthesis of chalcone analogues with increased antileishmanial activity. Bioorg Med Chem 14:1538–1545
Chen M, Zhai L, Christensen SB, Theander TG, Kharazmi A (2001) Inhibition of fumarate reductase in Leishmania major and l-donovani by chalcones. Antimicrob Agents Chemother 45:2023–2029
Chibale K, Haupt H, Kendrick H, Yardley V, Saravanamuthu A, Fairlamb AH, Croft SL (2001) Antiprotozoal and cytotoxicity evaluation of sulfonamide and urea analogues of quinacrine. Bioorg Med Chem Lett 11:2655–2657
Coura JR, Galvão-Castro B, Grimaldi Júnior G (1987) Disseminated American cutaneous leishmaniasis in a patient with AIDS. Mem Inst Oswaldo Cruz 82:581–582
Croft SL, Coombs GH (2003) Leishmaniasis—current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol 19:502–508
Croft SL, Barrett MP, Urbina J (2005) Chemotherapy of trypanosomiases and leishmaniasis. Trends Parasitol 21:508–512
De Benedetti PG, Fanelli F (2009) Ligand–receptor communication and drug design. Curr Protein Pept Sci 10:186–193
Ferreira VF, Jorqueira A, Souza AM, da Silva MN, de Souza MC, Gouvea RM, Rodrigues CR, Pinto AV, Castro HC, Santos DO, Araujo HP, Bourguignon SC (2006) Trypanocidal agents with low cytotoxicity to mammalian cell line: a comparison of the theoretical and biological features of lapachone derivatives. Bioorg Med Chem 15:5459–5466
Guru PY, Agrawal AK, Singha UK, Singhal A, Gupta CM (1989) Drug targeting in Leishmania donovani infections using tuftsin-bearing liposomes as drug vehicles. FEBS Lett 245:204–208
Hsieh HK, Lee TH, Wang JP, Wang JJ, Lin CN (1998) Synthesis and anti-inflammatory effect of chalcones and related compounds. Pharm Res 15:39–46
Lunardi F, Guzela M, Rodrigues AT, Correa R, Eger-Mangrich I, Steindel M, Grisard EC, Assreuy J, Calixto JB, Santos AR (2003) Trypanocidal and leishmanicidal properties of substitution-containing chalcones. Antimicrob Agents Chemother 47:1449–1451
Mueller M, Ritmeijer K, Balasegaram M, Koummuki Y, Santana MR, Davidson R (2007) Unresponsiveness to AmBisome in some Sudanese patients with kata-azar. Trans R Soc Trop Med Hyg 101:19–24
Ni L, Meng CQ, Sikorski JA (2004) Recent advances in therapeutic chalcones. Expert Opin Ther Pat 14:1669–1691
Nielsen SF, Boesen T, Larsen M, Schonning K, Kromann H (2004) Antibacterial chalcones–bioisosteric replacement of the 4′-hydroxy group. Bioorg Med Chem 12:3047–3054
Owa T, Okauchi T, Yoshimatsu K, Sugi NH, Ozawa Y, Nagasu T, Koyanagi N, Okabe T, Kitoh K, Yoshino H (2000) A focused compound library of novel N-(7-indolyl)benzenesulfonamides for the discovery of potent cell cycle inhibitors. Bioorg Med Chem Lett 10:1223–1226
Santos DO, Coutinho CER, Madeira MF, Gruszkowski CCB, Vieira RT, Nascimento SB, Bernardino AMR, Bourguignon SC, Côrtes-Real S, Pinho RT, Rodrigues CR, Castro HC (2008) Leishmaniasis treatment, a challenge that remains: a review. Parasitol Res 103:1–10
Shaha C (2006) Apoptosis in Leishmania species and its relevance to disease pathogenesis. Ind J Med Res 123:233–244
Singh S, Sivakumar R (2004) Challenges and new discoveries in the treatment of leishmaniasis. J Infect Chemother 10:307–315
Soong L, Duboise SM, Kima P, McMahon-Pratt D (1995) Leishmania pifanoi amastigote antigens protect mice against cutaneous leishmaniasis. Infect Immun 63:3559–3566
Trouiller P, Olliaro P, Torreele E, Orbinski J, Laing R, Ford N (2002) Drug development for neglected diseases: a deficient market and a public-health policy failure. Lancet 359:2188–2194
Uchiumi F, Hatano T, Ito H, Yoshida T, Tanuma SI (2003) Transcriptional suppression of the HIV promoter by natural compounds. Antiviral Res 58:89–98
World Health organization (WHO)—background information. http://www.who.int/leishmaniasis/en/. Accessed 12 Oct 2008a
World Health Organization (WHO)—control of neglected diseases. http://www.who.int/neglected_diseases/diseases/en/. Accessed 12 Oct 2008b
World Health organization (WHO)—seventeenth programme report of the UNICEF/UNDP/World Bank/WHO special programme for research and training in tropical diseases, Tropical disease research: progress 2005–2006. http://www.who.int/tdr/diseases/leish/default.htm. Accessed 12 Oct 2008c
Wu JH, Wang XH, Yi YH, Lee KH (2003) Anti-AIDS agents 54. A potent anti-HIV chalcone and flavonoids from genus Desmos. Bioorg Med Chem Lett 13:1813–1815
Acknowledgments
The authors thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico/CNPq (Brazil), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Universidade Federal Fluminense (UFF) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Souza, A.M.T., Castro, H.C., Brito, M.A. et al. Leishmania amazonensis Growth Inhibitors: Biological and Theoretical Features of Sulfonamide 4-Methoxychalcone Derivatives. Curr Microbiol 59, 374–379 (2009). https://doi.org/10.1007/s00284-009-9447-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-009-9447-2