Abstract
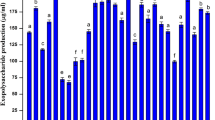
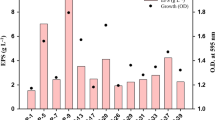
In the rhizosphere, exopolymers are also known to be useful to improve the moisture-holding capacity. The ability of the isolates from coastal sand dunes to produce exopolymers was determined. Among which the isolate, showing very high production of exopolysaccharide (EPS), Microbacterium arborescens––AGSB, a facultative alkalophile was further studied for exopolymer production. The isolate a gram-positive non-spore forming, slender rod, catalase positive, oxidase negative, showed growth in 12% sodium chloride. The culture was found to produce exopolymer which showed good aggregation of sand which has an important role in the stabilization of sand dunes. The exopolymer was further analysed. The cold isopropanol precipitation of dialysed supernatants grown in polypeptone yeast extract glucose broth produced partially soluble EPSs with glucose as the sole carbon source. Chemical analysis of the EPS revealed the presence of rhamnose, fucose, arabinose, mannose, galactose and glucose. On optimization of growth parameters (sucrose as carbon source and glycine as nitrogen source), the polymer was found to be a heteropolysaccharide containing mannose as the major component. It was interesting to note that the chemical composition of the exopolymers produced from both unoptimized and optimized culture conditions of Microbacterium arborescens––AGSB is different from those of other species from the same genera. This study shows that marine coastal environments such as coastal sand dunes, are a previously unexplored habitat for EPS-producing bacteria, and that these molecules might be involved in ecological roles protecting the cells against dessication especially in nutrient-limited environments such as the coastal sand dunes more so in the extreme conditions of pH. Such polysaccharides may help the bacteria to adhere to solid substrates and survive during the nutrient limitations.



Similar content being viewed by others
References
Desai KN, Untawale AG (2002) Sand dune vegetation of Goa; Conservation and Management. Botanical Society of Goa, Goa
Decho AW, Lopez GR (1993) Exopolymer microenvironments of microbial flora. Limnol Oceanog 38:1633–1645
Read RR, Costerton JW (1987) Purification and characterization of adhesive exoplysaccharides from Pseudomonas putida and Pseudomonas fuorescens. Can J Microbiol 33:1080–1090
Matsuyama H, Kawasaki K, Yumoto I, Shida O (1999) Microbacterium kitamiense sp. nov., a new polysaccharide-producing bacterium isolated from the wastewater of a sugar-beet factory. Int J Syst Bacteriol 49:1353–1357
Guezennec J (2002) Deep sea hydrothermal vents: a new source of innovative bacterial exopolysaccharides of biotechnological interest? J Indust Microbiol Biotechnol 29:204–208
Wotton RS (2004) The ubiquity and many roles of exopolymers (EPS) in aquatic systems. Sci Mar 68:13–21
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Bhosle NB, Sawant SS, Garg A, Wagh AB (1995) Isolation and partial chemical analysis of exopolysaccharides from a marine fouling diatom Navicula subinfecta. Bot Marina 38:103–110
Decho AW (1990) Microbial exopolymer secretions in ocean environment, their roles in food webs and marine processes. Oceanog Mar Biol Annu Rev 28:73–153
Morales BOO, Santiago-García JL, Chan-Bacab MJ, Moppert X, Miranda-Tello E, Fardeau ML, Carrero JC, Bartolo-Pérez P, Valadéz-González A, Guezennec J (2007) Characterization of extracellular polymers synthesized by tropical intertidal biofilm bacteria. J Appl Microbiol 102:254–264
Filisetti-cozzi TM, Nicholas CC (1991) Measurements of uronic acid without interference from neutral sugar. Anal Chem 197:157–162
Lowry OH, Nira J, Rosenbrough A, Farr L, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Slonecker JH, Orentas DG (1962) Pyruvic acid, a unique component of an exocellular bacterial polysaccharide. Nature 194:478–479
Martin JP (1971) Decomposition and binding action of polysaccharides in soil. Soil Biol Chem 3:33–41
Forster SM (1979) Microbial aggregation of sand in an embryo dune system. Soil Biol Chem 11:537–543
Ko SH, Lee HS, Park SH, Lee HK (2000) Optimal conditions for the production of exopolysaccharide by marine microorganism Hahella chenjuensis. Biotechnol Bioproc Eng 5:181–185
Cerning J, Renard C, Thibault JF, Bouillanne C, Landon M, Desmazeaud M, Toposirovic L (1994) Carbon source requirements for exopolysaccharide production by Lactobacillus casei CG11 and partial structure analysis of the polymer. Appl Environ Microbiol 60:3914–3919
Ashraf M, Berge O, Azam F, Heulin T (1999) Bacterial productivity of salt affected sites: I diversity of exopolysaccharide producing bacteria isolated from the rhizosphere of wheat (Triticum aesticum L.) grown in normal and saline Pakistani soils. Pak J Biol Sc 2:201–206
Kennedy AFD, Sutherland IW (1987) Analysis of bacterial exopolysaccharides. Biotechnol Appl Biochem 9:12–19
Bouchotroch S, Quesada E, Izquierdo I, Rodroguez M, Bejar V (2000) Bacterial exopolysaccharides produced by newly discovered bacteria belonging to the genus Halomonas, isolated from hypersaline habitats in Morocco. J Ind Microbiol Biotechnol 24:338–374
Corsaro MM, Lanzetta R, Parrilli E, Parrilli M, Tutino ML, Ummarino S (2004) Influence of growth temperature on lipid and phosphate contents of surface polysaccharides from the Antarctic bacterium Pseudoalteromonas haloplanktis TAC 125. J Bacteriol 186:29–34
Manca MC, Lama L, Improta R, Esposito E, Gambacorta A, Nicolaus B (1996) Chemical composition of two exopolysaccharides from Bacillus thermoantarcticus. Appl Environ Microbiol 62:3265–3269
Kenne L, Lindberg B (1983) Bacterial Polysaccharides. In: Aspinall GO (ed) The polysaccharides. Academic Press, New York, pp 287–363
Anton J, Meseguer I, Valera FR (1988) Production of an extracellular polysaccharide by Haloferax mediterranei. Appl Environ Microbiol 54:2381–2386
Mancuso NCA, Guezennec J, Bowman JP (2005) Bacterial exopolysaccharides from extreme marine environments with special consideration of the southern ocean, sea ice, and deep-sea hydrothermal vents: A Review. Mar Biotechnol 7:253–271
Acknowledgements
The authors thank the Ministry of Earth Sciences for the financial assistance provided for this research study. The authors further acknowledge the help rendered by Dr N B Bhosle, National Institute of Oceanography, Goa for the Gas chromatographic analysis of the polymers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Godinho, A.L., Bhosle, S. Sand Aggregation by Exopolysaccharide-Producing Microbacterium arborescens––AGSB. Curr Microbiol 58, 616–621 (2009). https://doi.org/10.1007/s00284-009-9400-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-009-9400-4