Abstract
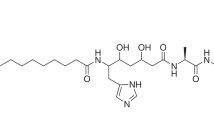
The marine bacterium “Pseudoalteromonas januaria” SUT 11 isolated from a seawater sample produced the rare cell-bound cyclic lipodepsipeptides A/A′, B/B′, and C/C′. The matrix-assisted laser desorption/ionization mass spectra indicated that one bromine atom presented in the peptides B/B′ and C/C′, whereas the component A/A′ contained no bromine atom. The acyldepsipeptides A/A′–C/C′ have an identical amino acid sequence, Thr-Val-Asn-Asn-Leu/allo-Ile, but differed in C-terminal amino acid and acyl moieties. Peptides A–C have Leu as a C-terminal amino acid, whereas peptides A′-C′ have allo-Ile. Acyl moieties in peptides A/A′, B/B′, and C/C′ have been found to consist of 11-(4′-hydroxyphenyl)-undeca-2,4,6,8,10-pentaenic acid, 9-(3′-bromo-4′-hydroxyphenyl)-nona-2,4,6,8-tetraenic acid, and 11-(3′-bromo-4′-hydroxyphenyl)-undeca-2,4,6,8,10-pentaenic acid, respectively. The structure of a main pair of peptides B/B′ with molecular masses 843/845 Da has been determined by means of ultraviolet, infrared, and two-dimensional nuclear magnetic resonance spectroscopy. We have demonstrated that tandem nano-electrospray ionization mass spectrometry is a very efficient way for the fast and sensitive investigation of lipopeptides A/A′ and C/C′ with molecular masses 791 and 869/871 Da, respectively, which have been isolated in small amounts.


Similar content being viewed by others
References
Barry AI (1980) Procedures and theoretical considerations for testing antimicrobial agents in agar media. In: Logan V (ed.) Antibiotics in laboratory medicine. William & Wilkins, Baltimore, pp 10–16
Felstenstein J (1993) PHYLIP (Phylogenetic inference package), Version 3.57c. University of Washington, Seattle
Gascuel O (1997) BIONJ: an improved version of the NJ algorithm based on a simple model of sequence data. Mol Biol Evol 14:685–695
HolmstrØm C, Kjelleberg CS (1999) Marine Pseudoalteromonas species are associated with higher organisms and produce biologically active extracellular agents. FEMS Microbiol Ecol 30:285–293
Isnansetyo A, Kamei Y (2003) MC21-A, a bactericidal antibiotic produced by a new marine bacterium, Pseudoalteromonas phenolica sp. nov. O-BC30T, against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 47:480–488
Ivanova EP, Kiprianova EA, Mikhailov VV, et al. (1996) Characterization and identification of marine Alteromonas nigrifaciens strains and emendation of the description. Int J Syst Bacteriol 46:223–228
Ivanova EP, Kiprianova EA, Mikhailov VV, et al. (1996) Phenotypic diversity of Pseudoalteromonas citrea from different marine habitats and emendation of the description. Int J Syst Bacteriol 48:247–256
Ivanova EP, Sawabe T, Lysenko AM, et al. (2002) Pseudoalteromonas ruthenica sp. nov., a new bacterium isolated from mussel. Int J Syst Evol Microbiol 52:235–240
Ivanova EP, Sawabe T, Alexeeva YV, et al. (2002) Pseudoalteromonas issachenkonii sp. nov., a bacterium that degrades the thallus of brown alga Fucus evanescens. Int J Syst Evol Microbiol 52:229–232
Ivanova EP, Gorshkova NM, Sawabe T, et al. (2004) Sulfitobacter delicatus sp. nov. and Sulfitobacter dubius sp. nov., respectively, from a starfish (Stellaster equestris) and sea grass (Zostera marina). Int J Syst Evol Microbiol 54:475–480
Ivanova EP, Flavier S, Christen R (2004) Phylogenetic relationships among marine Alteromonas-like proteobacteria: emendation of the family Alteromonadaceae and proposal of Pseudoalteromonadaceae fam. nov., Colwelliaceae fam. nov., Shewanellaceae fam. nov., Moritellaceae fam. nov., Ferrimonadaceae fam. nov., Idiomarinaceae fam. nov., Psychromonadaceae fam. nov. Int J Syst Evol Microbiol 54:1773–1788
Kates M (1986) Techniques in lipidology: Laboratory techniques in biochemistry and molecular biology, 2nd ed. Elsevier, Amsterdam
Laatsch H (2006) Marine bacterial metabolites. In: Proksch P, Müller WEG (Eds) Frontiers in marine biotechnology. Horizon Bioscience, Norfolk, UK, pp 225–288
Lovell FM (1966) The structure of a bromine-rich antibiotic. J Am Chem Soc 88:4510–4511
Marfey P (1984) Determination of d-amino acids. II. Use of a bifunctional reagent, 1,5-difluoro-2,4-dinitrobenzene. Carlsberg Res Commun 49:591–596
McCarthy SA, Johnson RM, Kakimoto D (1994) Characterization of an antibiotic produced by Alteromonas luteoviolacea Gauthier 1982, 85 isolated from Kinko Bay. Jpn J Appl Bacteriol 175:425–432
Perrière G, Gouy M (1998) WWW-Query: an on-line retrieval system for biological sequence banks. Biochimie 78:364–369
Shiozawa A, Kagasaki T, Torikata A, et al. (1995) Thiomarinol B and C, new antimicrobial antibiotics produced by a marine bacterium. J Antibiot 48:907–909
Shiozawa H, Shimada A, Takahashi S (1997) Thiomarinol D, E, F and G, new hybrid antimicrobial antibiotics produced by a marine bacterium: isolation, structure and antimicrobial activity. J Antibiot 50:449–452
Smibert RM, Krieg NR (1994) Phenotypic characterization. In: Gerhardt Ph, Murray RGE, Wood WA, Krieg NR (eds) Methods for general and molecular bacteriology. American Society for Microbiology, Washington, DC, pp 607–654
Speitling M (1998) Vergleich der metabolischen kapazität mariner und terristrischer mikroorganismen: isolierung und strukturaufklärung von Branimycin, Brom-alterochromid A/B und weiteren stoffwechselprodukten, PhD thesis, University of Goettingen
Teesch LM, Adams J (1991) Fragmentations of gas-phase complexes between alkali metal ions and peptides: metal ion binding to carbonyl oxygens and other neutral functional groups. J Am Chem Soc 113:812–820
Yoshikawa K, Takadera T, Adachi K, et al. (1997) Korormicin, a novel antibiotic specifically active against marine gram-negative bacteria, produced by a marine bacterium. J Antibiot 50:949–953
Acknowledgments
We thank Dr. G. Verbitskii (Far Eastern State University, Research and Educational Center of Basic Marine Biota Research) for the determination of the HPLC/ESI-MS data measured on an Agilent 1100 Series LC/MSD mass spectrometer (Hewlett Packard, USA). This work was partially supported by Russian Foundation for Basic Research grants 06-04-48578 and 05-04-48211, grants from Presidium of the Russian Academy of Sciences “Molecular and Cell Biology” 04-1-05-005, and a grant from president of Russian Federation “Leading Scientific School of RF” 6477.1006.4.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kalinovskaya, N.I., Dmitrenok, A.S., Kuznetsova, T.A. et al. “Pseudoalteromonas januaria” SUT 11 as the Source of Rare Lipodepsipeptides. Curr Microbiol 56, 199–207 (2008). https://doi.org/10.1007/s00284-007-9023-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-007-9023-6