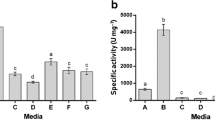
Abstract
Out of nine psychrotrophic bacterial strains isolated from cold environments of the Western Himalayas, SKPB5 was selected for protease purification and characterization because it had the largest zone of clearance on plate assay. On the basis of the phenotypic and biochemical characterization and 16S rRNA gene-sequencing studies, isolate was identified as Exiguobacterium sp. SKPB5. The protease was purified near to homogeneity with a purification fold of 7.1, and its molecular weight was determined to be 36 kDa. The enzyme exhibited maximum stability at 50°C and an optimal pH of 8.0. Metal ions Mg2+, Ca2+, Zn2+, and Mn2+ enhanced the enzyme activity, whereas Cu2+ had no effect. Phenylmethanesulfonyl fluoride and ethylenediaminetetraacetic acid did not show any effect on the activity of the enzyme, whereas a 20% increase in activity was observed when it was incubated in presence of reducing agents such as β-mercaptoethanol and dithiothreitol. This suggests that the protease isolated from psychrotrophic Exiguobacterium sp. SKPB5 belongs to the cysteine family. The results highlight the relevance of unexplored microbes from cold environments of Western Himalayas for the isolation of protease enzymes active at wide range of temperature and pH.





Similar content being viewed by others
Literature Cited
Baghel VS, Tripathi RD, Ramteke PW, Gopal K, Dwivedi S, Jain RK, Rai UN, Singh SN (2005) Psychrotrophic proteolytic bacteria from cold environment of Gangotri glacier, Western Himalaya, India, Enz Microbial Technol 36:654–6592
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal Biochem 7:248–254
Brenchley JE (1996) Psychrophilic microorganisms and their cold-active enzymes. J Ind Microbiol 17:432–437
Cowan TS, Steel KJ (1998) Manual-for-identifieation of-medieal-bacteria, ed. London: Cambridge University Press
Davail S, Feller G, Narinx E, Greday C (1994) Cold adaptation of proteins. Purification, characterization and sequencing of the heat-labile subtilisin from the Antarctic psychroplrile Bacillus; TA41. J BiorChem 269:17448–17453
Dube S, Singh L, Alam ST (2001) Proteolytic anaerobic bacteria from lake sediments of antarctica. Enz Microbial Technol 28:114–121
Fernandez J, Mohedano AF, Polanco MJ, Medina M, Nunez M (1996) Purification and characterization of an extracellullar cysteine protease produced by Micrpcoccus sp. INIA 528. J Appl Bacteriol 81:27–34
Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams ST (1994) Bergey’s manual of determinative bacteriology. 9th ed. Baltimore, MD: Williams and Wilkins
Kalisz MH (1988) Microbial proteases. Adv Biochem Eng Biotechnol 36:17–55
Kazan D, Denizci AA, Omer MNK, Erarslan A (2005) Purification and characterization of a serine alkaline protease from Bacillus clausi GMBAE 42. J Ind Microbiol Biotechnol 32:335–344
Kimura M (1980) A simple method for estimating evolutionary rates base substitution through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kristjansson MM, Magnusson OT, Magnusson HM, Alfredsson GA, Matsuzawa H (1999) Properties of a subtilisin-like proteinase from a psychrotrophic Vibrio species. Comparison to proteinase K and aqualysin I. Eur J Biochem 260:752–760
Kulakova L, Galkin A, Kurihara T, Yoshimura T, Esaki N (1999) Cold active serine alkaline protea.se from the psychrotrophic bacterium Shewanella strain ac 10: Gene cloning and enzyme purification and characterization. Appl Environ Microbiol 65:611–617
Kumar CG, Takagi H (1999) Microbial alkaline proteases: From a bioindustrial viewpoint. Biotechnol Adv 7:561–594
Kumar D, Bhalla TC (2004) Purification and characterization small size protease from Bacillus sp. APR-4. Ind J Expn Biol 42:515–521
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of the baeteriophage T4. Nature 227:685–689
Manachini PL, Fortina MG, Parini C (1988) Thermostable alkaline protease produced by Bacillus thermorubber, new sp. of Bacillus. Appl Microbiol Biotechnol 28:409–413
Margesin R, Schinner F (1994) Properties of cold adapted microorganism and their potential role in biotechnology. J Biotechnol 33:1–14
Morita RY (1975) Psychrophilic bacteria. Bact Rev 39:144–167
Oh KH, Seong CS, Lee SW, Kwon OS, Park YS (1999) Isolation of a psychrotrophic Azospirillum sp. and characterization of its extracellullar protease. FEMS Microbiol Lett 174:173–178
Outtrup H, Boyce COL (1990) Microbial proteinases and biotechnology. In: Fogarty WM, Kelly CT (eds) Microbial ezymes and biotechnology. New York: Elsevier Publishers. pp 227–254
Rao MB, Tanksli AM, Ghatgi MS, Deshpande VV (1998) Molecular and biotechnological aspects of microbiol proteases. Microbiol Mol Biol Rev 62:597–635
Rodrigues DF, Goris J, Vishnivetskaya T, Gilichinsky D, Thomashow MF, Tiedje JM (2006) Characterization of Exiguobacterium isolates from the Siberian permafrost Description of Exiguobacterium sibiricum sp. Nov. Extremophiles 10:285–294
Saitou N, Nei M (1987) The neighbor-joining method: A new method for reconstructing phylogenetic tree. Mol Biol Evol 4:406–425
Takami H, Akiba T, Horikoshi K (1990) Characterization of an alkaline protease from Bacillus sp. no. AH-101. Appl Microbiol Biotechnol 30:519–523
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: Improving the sensitivity of progressive multiple sequence weighing, position-specific gap penalties, and weight matrix choice. Nucleic Acids Res 22:4673–4680
Ulukanli Z, Digrak M (2002) Alkaliphilic micro-organisms and habitats. Turk J Biol 26:181–191
Acknowledgments
The authors are grateful to Dr. P.S. Ahuja, Director, Institute of Himalayan Bioresource Technology, Palampur, for valuable suggestions and encouragement during the experimental study. We also thank Harkeerat Atwal and Prashant Mohanpuria for extending help in various experiments. Council of Scientific and Industrial Research (CSIR) is duly acknowledged for providing financial support in the form of internal grants to Institute of Himalayan Bioresource Technology (IHBT). This IHBT communication number for this study is 642.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kasana, R.C., Yadav, S.K. Isolation of a Psychrotrophic Exiguobacterium sp. SKPB5 (MTCC 7803) and Characterization of Its Alkaline Protease. Curr Microbiol 54, 224–229 (2007). https://doi.org/10.1007/s00284-006-0402-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-006-0402-1