Abstract
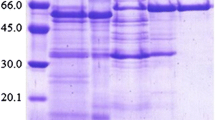
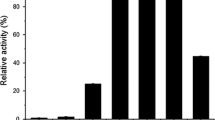
The mutant strain PN-120 of Cellulomonas flavigena produces a ß-glucosidase that is 10-fold more active than the corresponding enzyme isolated from the parental strain. These enzymes were partially purified through Q Sepharose and Bio-Gel filtration. A single protein band was detected on polyacrylamide–gel electrophoresis/zymogram using 4-methylumbelliferyl-β-D-glucoside. On sodium dodecyl sulfate–PAGE, the enzyme displayed three protein bands, suggesting that in C. flavigena the enzyme is oligomeric with a molecular mass of 210 kDa. On purification, the specific activity of ß-glucosidase isolated from PN-120 was increased 16-fold and showed three times more affinity for cellobiose than the enzyme of the parental strain; nevertheless, the optimum pH and temperature were similar for both enzymes. The kinetic parameters suggested that the increase in the activity of the enzyme, from the mutant strain, was caused by a mutation that affects the catalytic site of the enzyme. The partial amino-acid sequence of the isolated enzyme confirmed that it is a β-glucosidase because of its homology with other β-glucosidases produced by cellulolytic bacteria and fungi.


Similar content being viewed by others
Literature Cited
Bathia Y, Mishra S, Bisaria VS (2002) Microbial β-glucosidases: Cloning, properties, and applications. Crit Rev Biotechnol 22:375–407
Pack SP, Park K, Yoo YJ (2002) Enhancement of β-glucosidase stability and cellobiose-usage using surface-engineered recombinant Saccharomyces cerevisiae in ethanol production. Biotechnol Lett 24:1919–1925
Lynd LR, Weimer PJ, van Zyl WH, et al. (2002) Microbial cellulose utilization: Fundamentals and biotechnology. Microbiol Mol Biol Rev 3:506–577
Woodward J, Wiseman A (1982) Fungal and other β-glucosidases: Their properties and applications. Enzyme Microbiol Technol 4:73–79
Golias H, Dumsday GJ, Stanley GA, et al. (2000) Characteristics of cellulase preparations affecting the simultaneous saccharification and fermentation of cellulose to ethanol. Biotechnol Lett 22:617–621
Rajoka MI, Durrani IS, Khalid AM (2004) Kinetics of improved production and thermostability of an intracellular β-glucosidase from a mutant-derivative of Cellulomonas biazotea. Biotechnol Lett 26:281–285
De la Torre M, Casas C (1984) Isolation and characterization of a symbiotic cellulolytic mixed bacteria culture. Appl Microbiol Biotechnol 19:430–439
Ponce-Noyola T, de la Torre M (1995) Isolation of a high-specific-growth-rate mutant of Cellulomonas flavigena on sugar cane bagasse. Appl Microbiol Biotechnol 42:709–712
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Kim HK, Pack MY (1989) Cloning and expression of Cellulomonas fimi β-glucosidase genes in Escherichia coli. Enzyme Microbiol Technol 11:313–316
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268
Kinter M, Sherman NE (2000) Protein sequencing and identification using tandem mass spectrometry. New York, NY: Wiley Interscience
Ponce-Noyola T, de la Torre M (2001) Regulation of cellulases and xylanases from a depressed mutant of Cellulomonas flavigena growing on sugar-cane bagasse. Bioresource Technol 78:285–291
Henrissat B, Bairoch A (1996) Updating the sequence-based classification of glycosyl hydrolases. Biochem J 316:695–696
Siddiqui KS, Rashid MH, Ghauri TM, et al. (1997) Purification and characterization of an intracellular β-glucosidase from Cellulomonas biazotea. World J Microbiol Biotechnol 13:245–247
Matsui I, Sakai Y, Matsui E, et al. (2000) Novel substrate specificity of a membrane-bound β-glycosidase from the hyperthermophilic archeon Pyrococcus horikoshii. FEBS Lett 467:195–200
Acknowledgments
This work was supported by CONACyT-México(45678-Z). A. Barrera-Islas received a scholarship from CONACyT- México. The investigators thank O. Pérez-Avalos and M. Mercado-Morales for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barrera-Islas, G., Ramos-Valdivia, A., Salgado, L. et al. Characterization of a β-Glucosidase Produced by a High-Specific Growth-Rate Mutant of Cellulomonas flavigena . Curr Microbiol 54, 266–270 (2007). https://doi.org/10.1007/s00284-006-0105-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-006-0105-7