Abstract
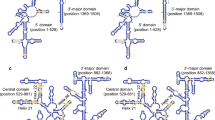
The ribosomal RNA molecule is an ideal model for evaluating the stability of a gene product under desiccation stress. We isolated 8 Nostoc strains that had the capacity to withstand desiccation in habitats and sequenced their 16S rRNA genes. The stabilities of 16S rRNAs secondary structures, indicated by free energy change of folding, were compared among Nostoc and other related species. The results suggested that 16S rRNA secondary structures of the desiccation-tolerant Nostoc strains were more stable than that of planktonic Nostocaceae species. The stabilizing mutations were divided into two categories: (1) those causing GC to replace other types of base pairs in stems and (2) those causing extension of stems. By mapping stabilizing mutations onto the Nostoc phylogenetic tree based on 16S rRNA gene, it was shown that most of stabilizing mutations had evolved during adaptive radiation among Nostoc spp. The evolution of 16S rRNA along the Nostoc lineage is suggested to be selectively advantageous under desiccation stress.


Similar content being viewed by others
Literature Cited
Auffinger P, Westhof E (1998) RNA base pair hydration. J Biomol Struct Dyn 16:693–707
Auffinger P, Westhof E (2000) Water and ion binding round RNA and DNA (C,G) oligomers. J Mol Biol 300:1113 J Biomol Struct Dynam 1131
Banerjee T, Gupta SK, Ghosh TC (2005) Role of mutational bias and natural selection on genome-wide nucleotide bias in prokaryotic organisms. Biosystems 81:11–18
Caetano-Anolles G (2002) Evolved RNA secondary structure and the rooting of the universal tree of life. J Mol Evol 54:333–345
Dodds WK, Gudder DA (1995) The ecology of Nostoc. J Phycol 31:2–18
Dvornyk V, Vinogradova O, Nevo E (2003) Origin and evolution of circadian clock genes in prokaryotes. Proc Natl Acad Sci U S A 100:2495–2500
Egli M, Portmann S, Usman N (1996) RNA hydration: A detailed look. Biochemistry 259:221–242
Feese E (1962) On the evolution of base composition of DNA. J Theor Biol 3:82–101
Galtier N, Gouy M, Gautier C (1996) SeaView and Phylo-win, two graphic tools for sequence alignment and molecular phylogeny. Comput Appl Biosci 12:543–548
Galtier N, Lobry JR (1997) Relationship between genomic G+C content, RNA secondary structures and optimal growth temperature in prokaryotes. J Mol Evol 44:632–636
Gugger M, Lyra C, Henriksen P, et al. (2002) Phylogenetic comparison of the cyanobacterial genera Anabaena and Aphanizomenon. Int J Syst Evol Microbiol 52:1867–1880
Gutell RR, Larsen N, Woese C (1994) Lessons from an evolving rRNA: 16S and 23S rRNA structures from a comparative perspective. Microbiol Rev 58:10–26
Hirai M, Yamakawa R, Nishio J, et al. (2004) Deactivation of photosynthetic activities is triggered by loss of a small amount of water in a desiccation-tolerant cyanobacterium, Nostoc commune. Plant Cell Physiol 45:872–878
Iteman I, Rippka R, Tandeau de Marsac N, et al. (2002) rDNA analyses of planktonic heterocystous cyanobacteria, including members of the genera Anabaenopsis and Cyanospira. Microbiology 148:481–496
Jäger K, Potts M (1988) Distinct fractions of genomic DNA from cyanobacterium Nostoc commune that differ in the degree of methylation. Gene 74:197–201
Khachane AN, Timmis KN, Martins dos Santos AP (2005) Uracil content of 16S rRNA of thermophilic and psychrophilic prokaryotes correlates inversely with their optimal growth temperatures. Nucleic Acids Res 33:4016–4022
Komárek J, Anagnostidis K (1989) Modern approach to the classification system of Cyanophytes 4-Nostocales. Arch Hydrobiol (Suppl. 82) 56:247–345
Lambert JD, Moran NA (1998) Deleterious mutations destabilize ribosomal RNA in endosymbiotic bacteria. Proc Natl Acad Sci U S A 95:4458–4462
Mathews DH, Sabina J, Zuker M, et al. (1999) Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary Structure. J Mol Biol 288:911–940
Mathews DH, Disney MD, Childs JL, et al. (2004) Incorporating chemical modification constraints into a dynamic programming algorithm for prediction of RNA secondary structure. Proc Natl Acad Sci U S A 101:7287–7292
Musto H, Naya H, Zavala A, et al. (2004) Correlations between genomic GC levels and optimal growth temperatures in prokaryotes. FEBS Lett 573:73–77
Naya H, Romero H, Zavala A, et al. (2002) Aerobiosis increases genomic GC% in prokaryotes. J Mol Evol 55:260–264
Nissen P, Hansen J, Ban N, et al. (2000) The structural basis of ribosome activity in peptide bond synthesis. Science 289:920–930
Paz A, Mester D, Baca I, et al. (2004) Adaptive role of increased frequency of polypurine tracts in mRNA sequences of thermophilic prokaryotes. Proc Natl Acad Sci U S A 101:2951–2956
Posada D, Crandall KA (1998) Modeltest: Testing the model of DNA substitution. Bioinformatics 14:817–818
Postius C, Ernst A (1999) Mechanisms of dominance: Coexistence of picocyanobacterial genotypes in freshwater ecosystem. Arch Microbiol 172:68–75
Potts M (1999) Mechanism of desiccation tolerance in cyanobacteria. Eur J Phycol 34:319–328
Rozners E, Moulder J (2004) Hydration of short DNA, RNA and 2’-Ome oligonucleotides determined by osmotic stressing. Nucleic Acids Res 32:248–254
Schultes EA, Hraber PT, LaBean TH (1999) Estimation the contributions of selection and self-organization in RNA secondary structure. J Mol Evol 49:76–83
Shirkey B, Kovark DP, Wright DJ, et al. (2000) Active Fe-containing superoxide dismutase and abundant sodF mRNA in Nostoc commune (Cyanobacteria) after years of desiccation. J Bacteriol 182:189–197
Shirkey B, McMaster NJ, Smith SC, et al. (2003) Genomic DNA of Nostoc commune (Cyanobacteria) becomes covalently modified during long-term (decades) desiccation but is protected from oxidative damage and degradation. Nucleic Acids Res 31:2995–3005
Sueoka N (1962) On the genetic basis of variation and heterogeneity of DNA base composition. Proc Natl Acad Sci U S A 85:2653–2657
Sueoka N (1992) Directional mutation pressure, selective constraints, and genetic equilibria. J Mol Evol 34:95–114
Stomp M, Huisman J, De Jongh F, et al. (2004) Adaptive divergence in pigment composition promotes phytoplankton biodiversity. Nature 432:104–107
Swofford DL (2001) PAUP* 4.0: Phylogenetic analysis using parsimony (*and other methods). Sunderland: Sinauer Associates
Tamaru Y, Takani Y, Yoshida T, et al. (2005) Crucial role of extracellular polysaccharides in desiccation and freezing tolerance in the terrestrial cyanobacterium Nostoc commune. Appl Environ Microbiol. 71:7327–7333
Thompson JD, Gibson TJ, Plewniak F, et al. (1997) The ClustalX windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 24:4876–4882
Whitton BA, Potts M (eds) (1999) Ecology of cyanobacteria: Their diversity in time and space. Dordret, The Netherlands: Kluwer Academic
Wild K, Sinning I, Cusack S (2001) Crystal structure of an early protein-RNA assembly complex of the signal recognition particle. Science 294:598–601
Wilmotte A, Van der Auwera G, De Wachter R (1993) Structure of the 16 S ribosomal RNA of the thermophilic cyanobacterium Chlorogloeopsis HTF (“Mastigocladus laminosus HTF’) strain PCC7518, and phylogenetic analysis. FEBS Lett 317:96–100
Wuyts J, Van de Peer Y, De Wachter R (2001) Distribution of substitution rates and location of insertion sites in the tertiary structure of ribosomal RNA. Nucleic Acids Res 29:5017–5028
Acknowledgments
This study was supported by the Chinese Natural Spark Program (2005EA173005). We thank the anonymous reviewers’ critical reading of the manuscript and helpful comments.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Han, D., Hu, Z. Mutations Stabilize Small Subunit Ribosomal RNA in Desiccation-Tolerant Cyanobacteria Nostoc . Curr Microbiol 54, 254–259 (2007). https://doi.org/10.1007/s00284-006-0095-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-006-0095-5