Abstract
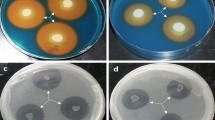
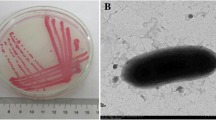
To test whether plant growth–promoting bacteria might be useful in facilitating the growth of Phragmites australis, the common reed, in the presence of metals and organic compounds, P. australis seeds were treated with plant growth–promoting bacteria. The bacterium Pseudomonas asplenii AC was genetically transformed to express a bacterial gene encoding the enzyme 1-aminocyclopropane-1-carboxylate deaminase, and both the native and transformed bacteria were tested in conjunction with P. australis. Inoculation of seeds, which were subsequently grown in the presence of copper or creosote, with transformed P. asplenii AC significantly increased seed germination. Moreover, the addition of either native or transformed P. asplenii AC to P. australis seeds enabled the plants (shoots and roots) to attain a greater size than noninoculated plants after growth in soil in the presence of either copper or creosote.



Similar content being viewed by others
Literature Cited
Abeles FB, Morgan PW, Saltveit ME Jr (1992) Ethylene in plant biology, 2nd ed. New York, NY: Academic
Ali NA, Bernal MP, Ater M (2002) Tolerance and bioaccumulation of copper in Phragmites australis and Zea mays. Plant Soil 239:103–111
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Burd GI, Dixon DG, Glick BR (2000) Plant growth-promoting bacteria that decrease heavy metal toxicity in plants. Can J Microbiol 46:237–245
Burke DJ, Hamerlynck EP, Hahn D (2002) Interactions among plant species and microorganisms in salt marsh sediments. Appl Environ Microbiol 69:1157–1164
Cunningham SD, Ow DW (1996) Promises and prospects of phytoremediation. Plant Physiol 110:715–719
Dworkin DN, Foster J (1958) Experiments with some microorganisms which utilize ethane and hydrogen. J Bacteriol 75:592–601
Glass DJ (2000) Economic potential of phytoremediation. In: Raskin I (ed) Phytoremediation of toxic metals: Using plants to clean up the environment. New York, NY: Wiley-Interscience, pp 15–31
Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 41:109–117
Glick BR, (1995) Metabolic load and heterogeneous gene expression. Biotechnol Adv 13:247–261
Glick BR, Penrose DM, Li J (1998) A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J Theor Biol 1990:63–68
Glick BR, Patten CL, Holguin G, Penrose DM (1999) Biochemical and genetic mechanisms used by plant growth promoting bacteria. London, UK: Imperial College Press, pp 86–179
Glickmann E, Dessaux Y (1995) A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol 61:793–796
Gordon SA, Weber RP (1951) Colorometric estimation of indoleacetic acid. Plant Physiol 26:192–195
Grichko VP, Glick BR (2001) Amelioration of flooding stress by ACC deaminase-containing plant growth-promoting bacteria. Plant Physiol Biochem 39:11–17
Holguin G, Glick BR (2001) Expression of the ACC deaminase gene from Enterobacter cloacae UW4 in Azospirillum brasilense. Microb Ecol 41:281–288
Holguin G, Glick BR (2003) Transformation of Azospirillum brasilense Cd with an ACC deaminase gene from Enterobacter cloacae UW4 fused to the Tetr gene promoter improves its fitness and plant growth promoting ability. Microb Ecol 4:122–133
Honma M, Shimomura T (1978) Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agric Biol Chem 42:1825–1831
Huang XD, El-Alawi Y, Penrose DM, Glick BR, Greenberg BM (2004) Responses of three grass species to creosote during phytoremediation. Environ Pollut 130:453–463
Koch B, Worm J, Jensen LE, Højberg O, Nybroe O (2001) Carbon limitation induces σs-dependent gene expression in Pseudomonas fluorescens in soil. Appl Environ Microbiol 67:3363–3370
Macek T, Mackovà M, Kás J (2000) Exploitation of plants for the removal of organics in environmental remediation. Biotechnol Adv 18:23–34
Massacci A, Pietrini F, Iannelli MA (2001) Remediation of wetlands by Phragmites australis: The biological basis. Minerva Biotechnologica 13:135–140
Mayak S, Tirosh T, Glick BR (2004) Plant growth promoting bacteria that confer resistance to water stress in tomato and pepper. Plant Sci 166:525–530
Mayak S, Tirosh T, Glick BR (2004). Plant growth-promoting bacteria that confer resistance in tomato to salt stress. Plant Physiol Biochem 42:565–572
Nie L, Shah S, Burd GI, Dixon DG, Glick BR (2002) Phytoremediation of arsenate contaminated soil by transgenic canola and the plant growth-promoting bacterium Enterobacter cloacae CAL2. Plant Physiol Biochem 40:355–361
Patten CL, Glick BR (2002) Regulation of indoleacetic acid production in Pseudomonas putida GR12-2 by tryptophan and the stationary phase sigma factor RpoS. Can J Microbiol 48:635–642
Patten CL, Glick BR (2002) The role of bacterial indoleacetic acid in the development of the host plant root system. Appl Environ Microbiol 68:3795–3801
Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant 118:10–15
Penrose DM, Moffatt BA, Glick BR (2001) Determination of 1-aminocyclopropane-1-carboxylic acid (ACC) to assess the effect of ACC deaminase-containing bacteria on roots of canola seedlings. Can J Microbiol 47:77–80
Saleh SS, Glick BR (2001) Involvement of gacS and rpoS in enhancement of the plant growth-promoting capabilities of Enterobacter cloacae CAL2 and UW4. Can J Microbiol 47:698–705
Shah S, Li J, Moffatt BA, Glick BR (1998) Isolation and characterization of ACC deaminase genes from different plant growth-promoting rhizobacteria. Can J Microbiol 44:833–843
Shardendu, Salhani N, Boulyga SF, Steugel E (2003) Phytoremediation of selenium by two halophyte species in subsurface flow constructed wetland. Chemosphere 50:967–973
Stoltz E, Greger M (2002) Accumulation properties of As, Cd, Cu, Pb and Zn by four wetland plant species growing on submerged mine tailings. Environmental and Experimental Botany 47:271–280
Tischer S, Hübner T (2002) Model trials for phytoremediation of hydrocarbon contaminated sites. International Journal of Phytoremediation 4:187–203
Weber H, Polen T, Heuveling J, Wendisch VF, Hengge R (2005) Genome-wide analysis of the general stress response network in Escherichia coli: σs-dependent genes, promoters, and sigma factor selectivity. J Bacteriol 187:1591–1603
Weis JS, Weis P (2004) Metal uptake, transport and release by wetland plants: Implications for phytoremediation and restoration. Environ Int 20:685–700
Xu KD, Franklin MJ, Park C-H, McFeters GA, Stewart PS (2001). Gene expression and protein levels of the stationary phase sigma factor, RpoS, in continuously-fed Pseudomonas aeruginosa biofilms. FEMS Microbiol Lett 199:67–71
Ye ZH, Baker AJM, Wong MH, Willis AJ (1997) Zinc, lead and cadmium tolerance, uptake and accumulation by the common reed, Phragmites australis (Cav) Trin Ex. Steudel. Ann Bot (Lond) 80:363–370
Acknowledgments
This work was supported by a Strategic Grant from the Natural Sciences and Engineering Research Council to B. R. G.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reed, M., Warner, B.G. & Glick, B.R. Plant Growth–Promoting Bacteria Facilitate the Growth of the Common Reed Phragmites australisin the Presence of Copper or Polycyclic Aromatic Hydrocarbons. Curr Microbiol 51, 425–429 (2005). https://doi.org/10.1007/s00284-005-4584-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-005-4584-8