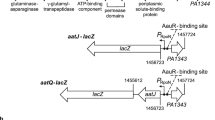
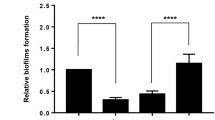
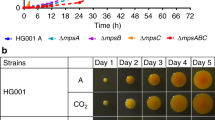
Abstract
The requirement for the mobA gene in key assimilatory and respiratory nitrogen metabolism of Pseudomonas aeruginosa PAO1 was investigated by mutational analysis of PA3030 (mobA; MoCo guanylating enzyme), PA1779 (nasA; assimilatory nitrate reductase), and PA3875 (narG; respiratory nitrate reductase). The mobA mutant was deficient in both assimilatory and respiratory nitrate reductase activities, whereas xanthine dehydrogenase activity remained unaffected. Thus, P. aeruginosa requires both the molybdopterin (MPT) and molybdopterin guanine dinucleotide (MGD) forms of the molybdenum cofactor for a complete spectrum of nitrogen metabolism, and one form cannot substitute for the other. Regulation studies using a Φ(PA3030-lacZGm) reporter strain suggest that expression of mobA is not influenced by the type of nitrogen source or by anaerobiosis, whereas assimilatory nitrate reductase activity was detected only in the presence of nitrate.

Similar content being viewed by others
Literature Cited
Cali BM, Micca JL, Stewart V (1989) Genetic-regulation of nitrate assimilation in Klebsiella pneumoniae M5al. J Bacteriol 171:2666–2672
Dias JM, Than ME, Humm A, Huber R, Bourenkov GP, Bartunik HD, et al. (1999) Crystal structure of the first dissimilatory nitrate reductase at 1.9 A solved by MAD methods. Structure Fold Des 7:65–79
Gutierrez JC, Ramos F, Ortner L, Tortolero M (1995) nasST, two genes involved in the induction of the assimilatory nitrite-nitrate reductase operon (nasAB) of Azotobacter vinelandii. Mol Microbiol 18:579–591
Johnson JL, Chaudhury M, Rajagopalan KV (1991) Identification of a molybdopterin-containing molybdenum cofactor in xanthine dehydrogenase from Pseudomonas aeruginosa. Biofactors 3:103–107
Johnson JL, Indermaur LW, Rajagopalan KV (1991) Molybdenum cofactor biosynthesis in Escherichia coli: Requirement of the chlB gene product for the formation of molybdopterin guanine dinucleotide. J Biol Chem 266:12140–12145
Johnson ME, Rajagopalan KV (1987) Involvement of chlA, E, M, and N Loci in Escherichia coli molybdopterin biosynthesis. J Bacteriol 169:117–125
Joshi MS, Rajagopalan KV (1994) Specific incorporation of molybdopterin in xanthine dehydrogenase of Pseudomonas aeruginosa. Arch Biochem Biophys 308:331–334
Lake MW, Temple CA, Rajagopalan KV, Schindelin H (2000) The crystal structure of the Escherichia coli MobA protein provides insight into molybdopterin guanine dinucleotide biosynthesis. J Biol Chem 275:40211–40217
Leimkuhler S, Kern M, Solomon PS, McEwan AG, Schwarz G, Mendel RR, et al. (1998) Xanthine dehydrogenase from the phototrophic purple bacterium Rhodobacter capsulatus is more similar to its eukaryotic counterparts than to prokaryotic molybdenum enzymes. Mol Microbiol 27:853–869
Leimkuhler S, Klipp W (1999) The molybdenum cofactor biosynthesis protein MobA from Rhodobacter capsulatus is required for the activity of molybdenum enzymes containing MGD, but not for xanthine dehydrogenase harboring the MPT cofactor. FEMS Microbiol Lett 174:239–246
Lin JT, Stewart V (1998) Nitrate assimilation by bacteria. Adv Microb Physiol 39:1–30
MacGregor CH, Schnaitman CA, Normansell DE (1974) Purification and properties of nitrate reductase from Escherichia coli K12. J Biol Chem 249:5321–5327
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory
Nichols J, Rajagopalan KV (2002) Escherichia coli MoeA and MogA–Function in metal incorporation step of molybdenum cofactor biosynthesis. J Biol Chem 277:24995–25000
Palmer T, Vasishta A, Whitty PW, Boxer DH (1994) Isolation of protein FA, a product of the mob locus required for molybdenum cofactor biosynthesis in Escherichia coli. Euro J Biochem 222:687–692
Pitterle DM, Rajagopalan KV (1993) The biosynthesis of molybdopterin in Escherichia coli purification and characterization of the converting factor. J Biol Chem 268:13499–13505
Richardson DJ, Berks BC, Russell DA, Spiro S, Taylor CJ (2001) Functional, biochemical and genetic diversity of prokaryotic nitrate reductases. Cell Mol Life Sci 58:165–178
Rivers SL, McNairn E, Blasco F, Giordano G, Boxer DH (1993) Molecular-genetic analysis of the moa operon of Escherichia coli K-12 required for molybdenum cofactor biosynthesis. Mol Microbiol 8:1071–1081
Rubio LM, Flores E, Herrero A (1999) Molybdopterin guanine dinucleotide cofactor in Synechococcus sp. nitrate reductase: identification of mobA and isolation of a putative moeB gene. FEBS Lett 462:358–362
Rudolph MJ, Wuebbens MM, Turque O, Rajagopalan KV, Schindelin H (2003) Structural studies of molybdopterin synthase provide insights into its catalytic mechanism. J Biol Chem 278:14514–14522
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press
Schindelin H, Kisker C, Rajagopalan KV (2001) Molybdopterin from molybdenum and tungsten enzymes. Adv Protein Chem 58:47–94
Schneider F, Lowe J, Huber R, Schindelin H, Kisker C, Knablein J (1996) Crystal structure of dimethyl sulfoxide reductase from Rhodobacter capsulatus at 1.88 A resolution. J Mol Biol 263:53–69
Schweizer HP (1992) Allelic exchange in Pseudomonas aeruginosa using novel ColE1-type vectors and a family of cassettes containing a portable OriT and the counter–selectable Bacillus subtilis sacB marker. Mol Microbiol 6:1195-1204
Schweizer HP (1993) 2 Plasmids, X1918 and Z1918, for easy recovery of the xylE and lacZ reporter genes. Gene 134:89–91
Sias SR, Ingraham JL (1979) Isolation and analysis of mutants of Pseudomonas aeruginosa unable to assimilate nitrate. Arch Microbiol 122:263-270
Simon R, Priefer U, Pühler A (1983) A broad-host range mobilization system for in vivo genetic engineering: Transposon mutagenesis in gram negative bacteria. Biotechnology 1:784–791
Vogel HJ, Bonner DM (1956) Acetylornithinase of Eschericia coli–Partial purification and some properties. J Biol Chem 218:97–106
Vollack KU, Xie J, Hartig E, Romling U, Zumft WG (1998) Localization of denitrification genes on the chromosomal map of Pseudomonas aeruginosa. Microbiology 144:441–448
Worlitzsch DR, Tarran M, Ulrich U, Schwab A, Cekici KC, Meyer P, et al. (2002) Reduced oxygen concentrations in airway mucus contribute to the early and late pathogenesis of Pseudomonas aeruginosa cystic fibrosis airway infection. J Clin Invest 103:317–325
Wuebbens MM, Liu MT, Rajagopalan K, Schindelin H (2000) Insights into molybdenum cofactor deficiency provided by the crystal structure of the molybdenum cofactor biosynthesis protein MoaC. Structure Fold Des 8:709-718
Wuebbens MM, Rajagopalan KV (2003) Mechanistic and mutational studies of Escherichia coli molybdopterin synthase clarify the final step of molybdopterin biosynthesis. J Biol Chem 278:14523–14532
Wuebbens MM, Rajagopalan KV (1995) Investigation of the early steps of molybdopterin biosynthesis in Escherichia coli through the use of in-vivo labeling studies. J Biol Chem 270:1082–1087
Acknowledgments
We thank Vandana Sharma for help with the experiments and Dr. Mary Connolly for help with the manuscript. This work was supported in part by the University of Dayton Summer Fellowship Program, Department of Biology, University of Dayton. Other support came from Public Health Service Grants from the National Institutes of Health (Grants. No. AI 40541 and GM 65873) to D. J. H.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Noriega, C., Hassett, D.J. & Rowe, J.J. The mobA Gene Is Required for Assimilatory and Respiratory Nitrate Reduction but not Xanthine Dehydrogenase Activity in Pseudomonas aeruginosa . Curr Microbiol 51, 419–424 (2005). https://doi.org/10.1007/s00284-005-0125-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-005-0125-8