Abstract
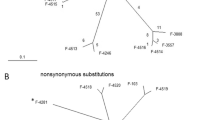
The random amplification of polymorphic DNA (RAPD) method was used to examine genetic variation in experimental clones of Pseudomonas pseudoalcaligenes in two experimental groups, as well as their common ancestor. Six clones derived from a single colony of P. pseudoalcaligenes were cultured in two different thermal regimes for 10 months. Three clones in the Control group were cultured at constant temperature of 35°C and another three clones in the High Temperature (HT) group were propagated at incremental temperature ranging from 41 to 47°C for 10 months. A total of 45 RAPD primers generated 146 polymorphic markers. Analysis of molecular variance (AMOVA) revealed mild (11%) but significant (P < 0.001) genetic difference between the Control and the HT clones. Phylogenetic analysis based on pairwise genetic distances showed that the HT clones were more divergent from the ancestor and from each other than the Control clones, implying that the HT clones of P. pseudoalcaligenes may have evolved faster than the Control clones.

Similar content being viewed by others
Literature Cited
FM Ausubel R Brent RE Kingston DD Moore JG Seidman JA Smith et al. (1992) Short protocols in molecular biology Greene Publishing Associates/Wiley New York
AF Bennett KM Dao RE Lenski (1990) ArticleTitleRapid evolution in response to high-temperature selection Nature 346 79–81 Occurrence Handle10.1038/346079a0 Occurrence Handle1:STN:280:By%2BA3cbpsl0%3D Occurrence Handle2195353
AF Bennett RE Lenski JE Mittler (1992) ArticleTitleEvolutionary adaptation to temperature. I. Fitness responses of Escherichia coli to changes in its thermal environments Evolution 46 16–30
AM Bronikowski AF Bennett RE Lenski (2001) ArticleTitleEvolutionary adaptation to temperature. VIII. Effects of temperature on growth rate in natural isolates of Escherichia coli and Salmonella enterica from different thermal environments Evolution 55 33–40 Occurrence Handle1:STN:280:DC%2BD3M7mt1Gmuw%3D%3D Occurrence Handle11263744
JJ Bull MR Badgett HA Wichman JP Huelsenbeck DM Hillis A Gulati et al. (1997) ArticleTitleExceptional convergent evolution in a virus Genetics 147 1497–1507 Occurrence Handle1:STN:280:DyaK1c%2Fnt1Cktg%3D%3D Occurrence Handle9409816
L Chao C Vargas BB Spear EC Cox (1983) ArticleTitleTransposable elements as mutator genes in evolution Nature 303 633–635 Occurrence Handle10.1038/303633a0 Occurrence Handle1:CAS:528:DyaL3sXks1Giur8%3D Occurrence Handle6304533
G Damiani P Amedeo C Bandi R Fani D Bellizzi V Sgaramella (1996) Bacteria identification by PCR-based techniques KW Adolph (Eds) Microbial genome methods CRC Press Boca Raton, FL 167–178
P Desjardins B Picard B Kaltenbock J Elion E Denamur (1995) ArticleTitleSex in Escherichia coli does not disrupt the clonal structure of the population: evidence from random amplified polymorphic DNA and restriction-fragment-length polymorphism J Mol Evol 41 440–448
L Excoffier PE Smouse JM Quattro (1992) ArticleTitleAnalysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data Genetics 131 479–491 Occurrence Handle1:CAS:528:DyaK38XlsVCntro%3D Occurrence Handle1644282
FW Fitch E Margoliash (1967) ArticleTitleConstruction of phylogenetic trees Science 155 279–284 Occurrence Handle1:CAS:528:DyaF2sXnt1Gnsw%3D%3D Occurrence Handle5334057
BG Hall (1999) ArticleTitleSpectra of spontaneous growth-dependent and adaptive mutations at ebgR J Bacteriol 181 1149–1155 Occurrence Handle1:CAS:528:DyaK1MXhsV2ru7c%3D Occurrence Handle9973340
AA Hoffmann PA Parsons (1991) Evolutionary genetics and environmental stress Oxford University Press New York
DR Huff R Peakall PE Smouse (1993) ArticleTitleRAPD variation within and among natural populations of outcrossing buffalograss [Buchole dactyloides (Nutt.) Engelm.] Theor Appl Genet 86 927–934
C Jaggi T Wirth B Baur (2000) ArticleTitleGenetic variability in subpopulations of the asp viper (Vipera aspis) in the Swiss Jura mountains: implications for a conservation strategy Biol Conserv 94 69–77 Occurrence Handle10.1016/S0006-3207(99)00162-7
RE Lenski AF Bennett (1993) ArticleTitleEvolutionary responses of Escherichia coli to thermal stress Am Nat 142 S47–S64 Occurrence Handle10.1086/285522
JA Lindsay (1995) ArticleTitleIs thermophily a transferable property in bacteria? Crit Rev Microbiol 21 165–174 Occurrence Handle1:STN:280:BymC3MvkvVc%3D Occurrence Handle8845061
SH Muller M Fischer (2001) ArticleTitleGenetic structure of the annual weed Senecio vulgaris in relation to habitat type and population size Mol Ecol 10 17–28 Occurrence Handle10.1046/j.1365-294X.2001.01169.x Occurrence Handle11251783
M Nei WH Li (1979) ArticleTitleMathematical model for studying genetic variation in terms of restriction endonucleases Proc Natl Acad Sci USA 76 5269–5273 Occurrence Handle1:CAS:528:DyaL3cXitVWn Occurrence Handle291943
D Paffetti C Scotti S Gnocchi S Fancelli M Bazzicalupo (1996) ArticleTitleGenetic diversity of an Italian Rhizobium meliloti population from different Medicago sativa varieties Appl Environ Microbiol 62 2279–2285
JA Rafalski (1997) Randomly amplified polymorphic DNA (RAPD) analysis G Caetano-Anolles PM Gresshoff (Eds) DNA markers Wiley-VCH New York 75–83
N Renders U Romling H Verbrugh A Van-Belkum (1996) ArticleTitleComparative typing of Pseudomonas aeruginosa by random amplification of polymorphic DNA or pulsed-field gel electrophoresis of DNA macrorestriction fragments J Clin Microbiol 34 3190–3195 Occurrence Handle1:CAS:528:DyaK2sXjs1Chtg%3D%3D Occurrence Handle8940470
MM Riehle AF Bennett AD Long (2001) ArticleTitleGenetic architecture of thermal adaptation in Escherichia coli Proc Natl Acad Sci USA 98 525–530 Occurrence Handle10.1073/pnas.021448998 Occurrence Handle1:CAS:528:DC%2BD3MXnslKhug%3D%3D Occurrence Handle11149947
MM Riehle AF Bennett RE Lenski AD Long (2003) ArticleTitleEvolutionary changes in heat-inducible gene expression in lines of Escherichia coli adapted to high temperature Physiol Genomics 24 47–58
N Saitou M Nei (1987) ArticleTitleThe neighbor-joining method: a new method for reconstructing phylogenetic trees Mol Biol Evol 4 406–425 Occurrence Handle1:STN:280:BieC1cbgtVY%3D Occurrence Handle3447015
B Shi X Xia (2003) ArticleTitleMorphological changes of Pseudomonas pseudoalcaligenes in response to temperature selection Curr Microbiol 46 120–123 Occurrence Handle10.1007/s00284-002-3824-4 Occurrence Handle1:CAS:528:DC%2BD3sXhtlOhs7k%3D Occurrence Handle12520367
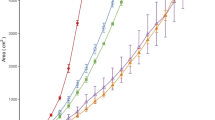
B Shi X Xia (2003) ArticleTitleChanges in growth parameters of Pseudomonas pseudoalcaligenes after ten months culturing at increasing temperature FEMS Microbiol Ecol 45 127–134 Occurrence Handle10.1016/S0168-6496(03)00129-6 Occurrence Handle1:CAS:528:DC%2BD3sXlsVaju70%3D
JWIM Simons (1982) ArticleTitleEffect of temperature on mutation in cultured human skin fibroblasts Mutat Res 92 417–426 Occurrence Handle1:STN:280:Bi2B3MbnvV0%3D Occurrence Handle7088011
J Wery B Hidayat JEA Kieboom (2001) ArticleTitleAn insertion sequence prepares Pseudomonas putida S12 for severe solvent stress J Biol Chem 276 5700–5706 Occurrence Handle10.1074/jbc.M007687200 Occurrence Handle1:CAS:528:DC%2BD3MXhs1Kmtrs%3D Occurrence Handle11094055
HA Wichman MR Badgett LA Scott CM Boulianne JJ Bull (1999) ArticleTitleDifferent trajectories of parallel evolution during viral adaptation Science 285 422–424 Occurrence Handle10.1126/science.285.5426.422 Occurrence Handle1:CAS:528:DyaK1MXkvVGgur0%3D Occurrence Handle10411508
JG Williams AR Kubelik KJ Livak JA Rafalski SV Tingey (1990) ArticleTitleDNA polymorphisms amplified by arbitrary primers are useful as genetic markers Nucleic Acids Res 18 6531–6535 Occurrence Handle1:CAS:528:DyaK3MXjslWmsA%3D%3D Occurrence Handle1979162
X Xia (2000) Data analysis in molecular biology and evolution Kluwer Academic Boston
X Xia T Wei Z Xie A Danchin (2002) ArticleTitleGenomic changes in nucleotide and dinucleotide frequencies in Pasteurella multocida cultured under high temperature Genetics 161 1385–1394 Occurrence Handle1:CAS:528:DC%2BD38XnsVCltbc%3D Occurrence Handle12196387
Acknowledgments
This study was supported by RGC grants from the Hong Kong Research Grant Council (HKU7265/00M, HKU7212/01M) to X.X. We thank S.G. Wu for providing the original strain, and R.B. Huey and A. Danchin for comments and discussion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, B., Xia, X. Genetic Variation in Clones of Pseudomonas pseudoalcaligenes After Ten Months of Selection in Different Thermal Environments in the Laboratory. Curr Microbiol 50, 238–245 (2005). https://doi.org/10.1007/s00284-004-4449-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-004-4449-6