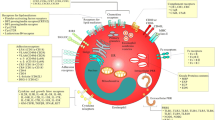
Abstract
Eosinophils comprise approximately 1–4% of total blood leukocytes that reside in the intestine, bone marrow, mammary gland, and adipose tissues to maintain innate immunity in healthy individuals. Eosinophils have four toxic granules known as major basic protein (MBP), eosinophil cationic protein (ECP), eosinophil peroxidase (EPO), and eosinophil-derived neurotoxin (EDN), and upon degranulation, these granules promote pathogenesis of inflammatory diseases like allergy, asthma, dermatitis, and gastrointestinal disorders. Additionally, the role of eosinophils is underscored in exocrine disorders including pancreatitis. Chronic pancreatitis (CP) is an inflammatory disorder that occurs due to the alcohol consumption, blockage of the pancreatic duct, and trypsinogen mutation. Eosinophil levels are detected in higher numbers in both CP and pancreatic cancer patients compared with healthy individuals. The mechanistic understanding of chronic inflammation–induced pancreatic malignancy has not yet been reached and requires further exploration. This review provides a comprehensive summary of the epidemiology, pathophysiology, evaluation, and management of eosinophil-associated pancreatic disorders and further summarizes current evidence regarding risk factors, pathophysiology, clinical features, diagnostic evaluation, treatment, and prognosis of eosinophilic pancreatitis (EP) and pancreatic cancer.





Similar content being viewed by others
References
Kay AB (2015) The early history of the eosinophil. Clin Exp Allergy 45(3):575–582
Fulkerson PC, Rothenberg ME (2018) Eosinophil development, disease involvement, and therapeutic suppression. Adv Immunol 138:1–34
Travers J, Rothenberg ME (2015) Eosinophils in mucosal immune responses. Mucosal Immunol 8(3):464–475
Straumann A, Safroneeva E (2012) Eosinophils in the gastrointestinal tract: friends or foes? Acta Gastroenterol Belg 75(3):310–315
Shukla A, Mishra A, Venkateshaiah SU, Manhoar M, Mahadevappa CP, Mishra A (2015) Elements involved in promoting eosinophilic gastrointestinal disorders. J Genet Syndr Gene Ther 6:265
Tian L et al (2016) Eosinophilic pancreatitis: three case reports and literature review. Mol Clin Oncol 4(4):559–562
Abraham SC, Leach S, Yeo CJ, Cameron JL, Murakata LA, Boitnott JK, Albores-Saavedra J, Hruban RH (2003) Eosinophilic pancreatitis and increased eosinophils in the pancreas. Am J Surg Pathol 27(3):334–342
Prussin C (2014) Eosinophilic gastroenteritis and related eosinophilic disorders. Gastroenterol Clin N Am 43(2):317–327
Venkateshaiah SU, Mishra A, Manohar M, Verma AK, Rajavelu P, Niranjan R, Wild LG, Parada NA, Blecker U, Lasky JA, Mishra A (2018) A critical role for IL-18 in transformation and maturation of naive eosinophils to pathogenic eosinophils. J Allergy Clin Immunol 142(1):301–305
Venkateshaiah SU, Manohar M, Verma AK, Blecker U, Mishra A (2016) Possible noninvasive biomarker of eosinophilic esophagitis: clinical and experimental evidence. Case Rep Gastroenterol 10(3):685–692
Petrov MS, Yadav D (2019) Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol 16(3):175–184
Colvin SD, Smith EN, Morgan DE, Porter KK (2020) Acute pancreatitis: an update on the revised Atlanta classification. Abdom Radiol (NY) 45(5):1222–1231
Manohar M, Verma AK, Venkateshaiah SU, Sanders NL, Mishra A (2017) Chronic pancreatitis associated acute respiratory failure. MOJ Immunology 5(2):1–7
Manohar M, Verma AK, Venkateshaiah SU, Sanders NL, Mishra A (2017) Pathogenic mechanisms of pancreatitis. World J Gastrointest Pharmacol Ther 8(1):10–25
Manohar M et al (2017) Significance of eosinophils in promoting pancreatic malignancy. J Gastroenterol Pancreatol Liver Disord:5(1)
Zheng L, Xue J, Jaffee EM, Habtezion A (2013) Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology 144(6):1230–1240
Manohar M, Verma AK, Venkateshaiah SU, Mishra A (2017) Immunological responses involved in promoting acute and chronic pancreatitis. J Clin Immunol Res 1:1–8
Juniper K Jr (1955) Chronic relapsing pancreatitis with associated marked eosinophilia and pleural effusion. Am J Med 19(4):648–651
Manohar M, Verma AK, Venkateshaiah SU, Mishra A (2018) Role of eosinophils in the initiation and progression of pancreatitis pathogenesis. American Journal of Physiology-Gastrointestinal and Liver Physiology 314(2):G211–G222
Xue J, Sharma V, Hsieh MH, Chawla A, Murali R, Pandol SJ, Habtezion A (2015) Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nat Commun 6:7158
Yuan BS, Zhu RM, Braddock M, Zhang XH, Shi W, Zheng MH (2007) Interleukin-18: a pro-inflammatory cytokine that plays an important role in acute pancreatitis. Expert Opin Ther Targets 11(10):1261–1271
Ueda T, Takeyama Y, Yasuda T, Matsumura N, Sawa H, Nakajima T, Ajiki T, Fujino Y, Suzuki Y, Kuroda Y (2006) Significant elevation of serum interleukin-18 levels in patients with acute pancreatitis. J Gastroenterol 41(2):158–165
Wereszczynska-Siemiatkowska U, Mroczko B, Siemiatkowski A (2002) Serum profiles of interleukin-18 in different severity forms of human acute pancreatitis. Scand J Gastroenterol 37(9):1097–1102
Dutt P, Shukla JS, Ventateshaiah SU, Mariswamy SJ, Mattner J, Shukla A, Mishra A (2015) Allergen-induced interleukin-18 promotes experimental eosinophilic oesophagitis in mice. Immunol Cell Biol 93(10):849–857
Verma AK, Kandikattu HK, Manohar M, Shukla A, Upparahalli Venkateshaiah S, Zhu X, Mishra A (2019) Intestinal overexpression of IL-18 promotes eosinophils-mediated allergic disorders. Immunology 157(2):110–121
McBrien CN, Menzies-Gow A (2017) The biology of eosinophils and their role in asthma. Front Med (Lausanne) 4:93
Hamann KJ, Barker RL, ten RM, Gleich GJ (1991) The molecular biology of eosinophil granule proteins. Int Arch Allergy Appl Immunol 94(1–4):202–209
Kandikattu HK, Venkateshaiah SU, Mishra A (2019) Synergy of interleukin (IL)-5 and IL-18 in eosinophil mediated pathogenesis of allergic diseases. Cytokine Growth Factor Rev 47:83–98
Varricchi G, Bagnasco D, Borriello F, Heffler E, Canonica GW (2016) Interleukin-5 pathway inhibition in the treatment of eosinophilic respiratory disorders: evidence and unmet needs. Curr Opin Allergy Clin Immunol 16(2):186–200
Mishra A, Hogan SP, Lee JJ, Foster PS, Rothenberg ME (1999) Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J Clin Invest 103(12):1719–1727
Kita H (2013) Eosinophils: multifunctional and distinctive properties. Int Arch Allergy Immunol 161(Suppl. 2):3–9
Johnston LK, Bryce PJ (2017) Understanding interleukin 33 and its roles in eosinophil development. Front Med (Lausanne) 4:51
Lacy P, Moqbel R (2000) Eosinophil cytokines. Chem Immunol 76:134–155
Spencer LA, Szela CT, Perez SA, Kirchhoffer CL, Neves JS, Radke AL, Weller PF (2009) Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J Leukoc Biol 85(1):117–123
Wen T, Rothenberg ME (2016) The regulatory function of eosinophils. Microbiol Spectr 4(5)
Iwasaki H, Mizuno SI, Mayfield R, Shigematsu H, Arinobu Y, Seed B, Gurish MF, Takatsu K, Akashi K (2005) Identification of eosinophil lineage-committed progenitors in the murine bone marrow. J Exp Med 201(12):1891–1897
Mori Y, Iwasaki H, Kohno K, Yoshimoto G, Kikushige Y, Okeda A, Uike N, Niiro H, Takenaka K, Nagafuji K, Miyamoto T, Harada M, Takatsu K, Akashi K (2009) Identification of the human eosinophil lineage-committed progenitor: revision of phenotypic definition of the human common myeloid progenitor. J Exp Med 206(1):183–193
Xia LX et al (2016) Eosinophil differentiation in the bone marrow is promoted by protein tyrosine phosphatase SHP2. Cell Death Dis 7:e2175
Uhm TG, Kim BS, Chung IY (2012) Eosinophil development, regulation of eosinophil-specific genes, and role of eosinophils in the pathogenesis of asthma. Allergy, Asthma Immunol Res 4(2):68–79
Blanchard C, Rothenberg ME (2009) Biology of the eosinophil. Adv Immunol 101:81–121
Mesnil C, Raulier S, Paulissen G, Xiao X, Birrell MA, Pirottin D, Janss T, Starkl P, Ramery E, Henket M, Schleich FN, Radermecker M, Thielemans K, Gillet L, Thiry M, Belvisi MG, Louis R, Desmet C, Marichal T, Bureau F (2016) Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest 126(9):3279–3295
Shi H-Z, Humbles A, Gerard C, Jin Z, Weller PF (2000) Lymph node trafficking and antigen presentation by endobronchial eosinophils. J Clin Invest 105(7):945–953
Akuthota P, Melo RCN, Spencer LA, Weller PF (2012) MHC class II and CD9 in human eosinophils localize to detergent-resistant membrane microdomains. Am J Respir Cell Mol Biol 46(2):188–195
Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, Lee NA, Lee JJ (2008) Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J Exp Med 205(3):699–710
Mishra A et al (2000) Peyer’s patch eosinophils: identification, characterization, and regulation by mucosal allergen exposure, interleukin-5, and eotaxin. Blood, The Journal of the American Society of Hematology 96(4):1538–1544
Beller A et al (2014) Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis. Immunity 40(4):582–593
Jung Y, Wen T, Mingler MK, Caldwell JM, Wang YH, Chaplin DD, Lee EH, Jang MH, Woo SY, Seoh JY, Miyasaka M, Rothenberg ME (2015) IL-1β in eosinophil-mediated small intestinal homeostasis and IgA production. Mucosal Immunol 8(4):930–942
Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM (2011) Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332(6026):243–247
Brigger D, Riether C, van Brummelen R, Mosher KI, Shiu A, Ding Z, Zbären N, Gasser P, Guntern P, Yousef H, Castellano JM, Storni F, Graff-Radford N, Britschgi M, Grandgirard D, Hinterbrandner M, Siegrist M, Moullan N, Hofstetter W, Leib SL, Villiger PM, Auwerx J, Villeda SA, Wyss-Coray T, Noti M, Eggel A (2020) Eosinophils regulate adipose tissue inflammation and sustain physical and immunological fitness in old age. Nature Metabolism 2(8):688–702
Marichal T, Mesnil C, Bureau F (2017) Homeostatic eosinophils: characteristics and functions. Front Med (Lausanne) 4:101
Mishra A, Hogan SP, Brandt EB, Wagner N, Crossman MW, Foster PS, Rothenberg ME (2002) Enterocyte expression of the eotaxin and interleukin-5 transgenes induces compartmentalized dysregulation of eosinophil trafficking. J Biol Chem 277(6):4406–4412
Mishra A, Hogan SP, Brandt EB, Rothenberg ME (2002) IL-5 promotes eosinophil trafficking to the esophagus. J Immunol 168(5):2464–2469
Mishra A, Hogan SP, Brandt EB, Rothenberg ME (2001) An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest 107(1):83–90
Aun MV et al (2017) Animal models of asthma: utility and limitations. J Asthma Allergy 10:293–301
Braun A, Tschernig T (2006) Animal models of asthma: innovative methods of lung research and new pharmacological targets. Exp Toxicol Pathol 57(Suppl 2):3–4
Mosmann TR, Coffman RL (1989) TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 7:145–173
Zosky GR, Sly PD (2007) Animal models of asthma. Clin Exp Allergy 37(7):973–988
Kandikattu HK, Venkateshaiah SU, Verma AK, Mishra A (2021) Tacrolimus (FK506) treatment protects allergen, IL-5, and IL-13-induced mucosal eosinophilia. Immunology
Mishra A (2013) Significance of mouse models in dissecting the mechanism of human eosinophilic gastrointestinal diseases (EGID). J Gastroenterol Hepatol Res 2(11):845–853
Hogan SP, Mishra A, Brandt EB, Royalty MP, Pope SM, Zimmermann N, Foster PS, Rothenberg ME (2001) A pathological function for eotaxin and eosinophils in eosinophilic gastrointestinal inflammation. Nat Immunol 2(4):353–360
Samlani Z, Lemfadli Y, Habiyaremye C (2020) Rare association of eosinophilic pancreatitis and ulcerative colitis: a case report and a review of the literature. Gastroenterol Hepatol Open Access 11(2):52–55
Bastid C, Sahel J, Choux R, Payan MJ, Sarles H (1990) Eosinophilic pancreatitis: report of a case. Pancreas 5(1):104–107
Lyngbaek S, Adamsen S, Aru A, Bergenfeldt M (2006) Recurrent acute pancreatitis due to eosinophilic gastroenteritis. Case report and literature review. JOP 7(2):211–217
Kakodkar S, Omar H, Cabrera J, Chi K (2015) Eosinophilic pancreatitis diagnosed with endoscopic ultrasound. ACG Case Rep J 2(4):239–241
Flejou JF, Potet F, Bernades P (1989) Eosinophilic pancreatitis: a rare manifestation of digestive allergy? Gastroenterol Clin Biol 13(8–9):731–733
Reppucci J, Chang M, Hughes S, Liu X (2017) Eosinophilic pancreatitis: a rare cause of recurrent acute pancreatitis. Case Rep Gastroenterol 11(1):120–126
Hashimoto F (1980) Transient eosinophilia associated with pancreatitis and pseudocyst formation. Arch Intern Med 140(8):1099–1100
Cay A, Imamoglu M, Cobanoglu U (2006) Eosinophilic pancreatitis mimicking pancreatic neoplasia. Can J Gastroenterol 20(5):361–364
Hayden DW, Kruiningen HJ (1975) Experimentally induced canine toxocariasis: laboratory examinations and pathologic changes, with emphasis on the gastrointestinal tract. Am J Vet Res 36(11):1605–1614
Petty DP, Lange AL, Verster A, Hattingh J (1992) Necropsies of eight horses infected with Strongylus equinus and Strongylus edentatus. J S Afr Vet Assoc 63(2):66–69
Novaes G, Cardozo Cde C, Costa NM, de Falco CN, de Carvalho MH, de Queiroz AC (1990) Experimental chronic interstitial pancreatitis induced by scorpion toxin in rats. Arq Gastroenterol 27(4):187–190
Vazquez Rodriguez JJ et al (1973) Pancreatitis and eosinophilic gastroenteritis. Int Surg 58(6):415–419
Maeshima A, Murakami H, Sadakata H, Saitoh T, Matsushima T, Tamura J, Karasawa M, Naruse T (1997) Eosinophilic gastroenteritis presenting with acute pancreatitis. J Med 28(3–4):265–272
Polyak S, Smith TA, Mertz H (2002) Eosinophilic gastroenteritis causing pancreatitis and pancreaticobiliary ductal dilation. Dig Dis Sci 47(5):1091–1095
Bellaiche G, Fontaine H, Choudat L, Lusina D, Ley G, Slama JL (1997) Pancreatic involvement, ascites and diarrhea in idiopathic hypereosinophilic syndrome. Gastroenterol Clin Biol 21(6–7):519–522
Eugene C et al (1984) Icterus disclosing pancreatic involvement in idiopathic hypereosinophilic syndrome. Gastroenterol Clin Biol 8(12):966–969
Rakesh K, Banerjee R, Gupta R, Ramji C, Pradeep R, Rao GV, Reddy DN (2007) Eosinophilic pancreatitis with pseudocyst. Indian J Gastroenterol 26(3):136–137
Baek MS, Mok YM, Han WC, Kim YS (2014) A patient with eosinophilic gastroenteritis presenting with acute pancreatitis and ascites. Gut Liver 8(2):224–227
Tse KY, Christiansen SC (2015) Eosinophilic gastroenteritis due to egg allergy presenting as acute pancreatitis. Allergy Rhinol (Providence) 6(1):80–81
Suzuki S, Homma T, Kurokawa M, Matsukura S, Adachi M, Wakabayashi K, Nozu F, Tazaki T, Kimura T, Matsuura T, Fukuda M, Shiozawa E, Takimoto M (2012) Eosinophilic gastroenteritis due to cow’s milk allergy presenting with acute pancreatitis. Int Arch Allergy Immunol 158(Suppl 1):75–82
Bass DA (1975) Behavior of eosinophil leukocytes in acute inflammation. II. Eosinophil dynamics during acute inflammation. J Clin Invest 56(4):870–879
Kedia S, Dhingra R, Garg PK (2013) Recurrent acute pancreatitis: an approach to diagnosis and management. Trop Gastroenterol 34(3):123–135
Testoni PA (2014) Acute recurrent pancreatitis: etiopathogenesis, diagnosis and treatment. World J Gastroenterol 20(45):16891–16901
Tokoo M, Oguchi H, Kawa S, Homma T, Nagata A (1992) Eosinophilia associated with chronic pancreatitis: an analysis of 122 patients with definite chronic pancreatitis. Am J Gastroenterol 87(4):455–460
Imbert Y et al (1984) Acute outbreaks of chronic pancreatitis associated with recurrent hypereosinophilia in patients with progressive lipodystrophy. Presse Med 13(30):1850
Wang Q, Lu CM, Guo T, Qian JM (2009) Eosinophilia associated with chronic pancreatitis. Pancreas 38(2):149–153
Manohar M, Kandikattu HK, Verma AK, Mishra A (2018) IL-15 regulates fibrosis and inflammation in a mouse model of chronic pancreatitis. American Journal of Physiology-Gastrointestinal and Liver Physiology 315(6):G954–G965
Manohar M (2017) Eosinophilic pancreatitis need an attention. J Pulmonol Clin Res 1(1):1–2
Keith RG, Keshavjee SH, Kerenyi NR (1985) Neuropathology of chronic pancreatitis in humans. Can J Surg 28(3):207–211
Hart PA, Zen Y, Chari ST (2015) Recent advances in autoimmune pancreatitis. Gastroenterology 149(1):39–51
Sah RP, Pannala R, Zhang L, Graham RP, Sugumar A, Chari ST (2010) Eosinophilia and allergic disorders in autoimmune pancreatitis. Am J Gastroenterol 105(11):2485–2491
Nikolic S, Brehmer K, Panic N, Valente R, Löhr JM, Vujasinovic M (2020) Cardiovascular and lung involvement in patients with autoimmune pancreatitis. J Clin Med 9(2):409
Barresi G, Inferrera C, De Luca F (1978) Eosinophilic pancreatitis in the newborn infant of a diabetic mother. Virchows Arch A Pathol Anat Histol 380(4):341–348
Payan H, Lebreuil G, Vague P (1968) Eosinophilic cell pancreatitis in newborn infants of diabetic mothers. Ann Anat Pathol (Paris) 13(1):87–96
George ER, Patel SS, Sen P, Sule N (2011) A unique case of eosinophilic pancreatitis and anencephaly in the fetus of a type I diabetic mother. Gastroenterology Res 4(4):174–176
Neuwirth A, Dobeš J, Oujezdská J, Ballek O, Benešová M, Šumník Z, Včeláková J, Koloušková S, Obermannová B, Kolář M, Štechová K, Filipp D (2012) Eosinophils from patients with type 1 diabetes mellitus express high level of myeloid alpha-defensins and myeloperoxidase. Cell Immunol 273(2):158–163
Moussa K, Gurung P, Adams-Huet B, Devaraj S, Jialal I (2019) Increased eosinophils in adipose tissue of metabolic syndrome. J Diabetes Complicat 33(8):535–538
Wu WY, Zhou XJ, Sun PP, Yu XJ, Wang SX, Qu L, Zhang F, Ma YY, Lv JC, Liu G, Yang L (2020) Interstitial eosinophilic infiltration in diabetic nephropathy is indicative of poor prognosis, with no therapy benefit from steroid. J Diabetes 12(12):881–894
Ibrahim U, Asti D, Saqib A, Mudduluru BM, Ayaz S, Odaimi M (2017) Eosinophilia as the presenting sign in pancreatic cancer: an extremely rare occurrence. Postgrad Med 129(3):399–401
Gitto SB et al (2020) Identification of a novel IL-5 signaling pathway in chronic pancreatitis and crosstalk with pancreatic tumor cells. Cell Communication and Signaling 18(1):1–14
Abu-Zaid A, Azzam A, Abudan Z, Algouhi A, Almana H, Amin T (2014) Sporadic pancreatic vasoactive intestinal peptide-producing tumor (VIPoma) in a 47-year-old male. Hematology/oncology and stem cell therapy 7(3):109–115
Schizas D, Mastoraki A, Bagias G, Patras R, Moris D, Lazaridis II, Arkadopoulos N, Felekouras E (2019) Clinicopathological data and treatment modalities for pancreatic vipomas: a systematic review. J BUON 24:415–423
Verma AK, Manohar M, Venkateshaiah SU, Blecker U, Collins MH, Mishra A (2018) Role of vasoactive intestinal peptide in promoting the pathogenesis of eosinophilic esophagitis (EoE). Cellular and Molecular Gastroenterology and Hepatology 5(1):99–100.e7
Verma AK, Manohar M, Upparahalli Venkateshaiah S, Mishra A (2017) Neuroendocrine cells derived chemokine vasoactive intestinal polypeptide (VIP) in allergic diseases. Cytokine Growth Factor Rev 38:37–48
Jang D (2018) Chronic myocarditis mimicking acute coronary syndrome due to hypereosinophilia which was produced by produced by pancreatic cancer. Case reports and Images 2:1–4
Descamps C, Billeret-Lebranchu V, Vankemmel M, Ernst O, Devred M, Lecomte-Houcke M, Colombel JF (1998) Eosinophilic pancreatitis: a difficult diagnosis. Gastroenterol Clin Biol 22(11):970–972
Hirata J, Koga T, Nishimura J, Ibayashi H (1987) Pancreatic carcinoma associated with marked eosinophilia: a case report. Eur J Haematol 39(5):462–466
Le Connie D, Nguyen H (2004) Eosinophilic gastroenteritis, ascites, and pancreatitis: a case report and review of the literature. South Med J 97(9):905–906
Javid Bhat K, Bhat S, Dutt K, Gupta S, Jeelani Samoon H (2014) Chronic diarrhea, eosinophilic ascites, acute pancreatitis and deep venous thrombosis: a case report. Caspian J Intern Med 5(3):182–185
Euscher E, Vaswani K, Frankel W (2000) Eosinophilic pancreatitis: a rare entity that can mimic a pancreatic neoplasm. Ann Diagn Pathol 4(6):379–385
Barthet M, Hastier P, M. Buckley MJ, Bernard JP, Sastre B, Baroni JL, Salducci J, Delmont J (1998) Eosinophilic pancreatitis mimicking pancreatic neoplasia: EUS and ERCP findings--is nonsurgical diagnosis possible? Pancreas 17(4):419–422
De Moura DTH et al (2019) A rare non-oncological pancreatic mass: eosinophilic pancreatitis diagnosis through EUS-FNA. Endosc Int Open 7(2):E151–E154
Pinte L, Baicus C (2019) Eosinophilic pancreatitis versus pancreatitis associated with eosinophilic gastroenteritis -a systematic review regarding clinical features and diagnosis. Rom J Intern Med 57:284–295
Rothenberg ME, Ownbey R, Mehlhop PD, Loiselle PM, van de Rijn M, Bonventre JV, Oettgen HC, Leder P, Luster AD (1996) Eotaxin triggers eosinophil-selective chemotaxis and calcium flux via a distinct receptor and induces pulmonary eosinophilia in the presence of interleukin 5 in mice. Mol Med 2(3):334–348
Fujimoto T, Imaeda H, Takahashi K, Nishida A, Shioya M, Inatomi O, Bamba S, Shiomi H, Tani M, Andoh A (2016) Eotaxin-3 (CCL26) expression in human pancreatic myofibroblasts. Pancreas 45(3):420–424
Stevens KJ, Lisanti C (2020) Pancreas imaging, in StatPearls: Treasure Island (FL)
Schmidt DN, Pandol SJ (1990) Differing effects of ethanol on in vitro stimulated pancreatic enzyme secretion in ethanol-fed and control rats. Pancreas 5(1):27–36
Ebeid AM, Murray PD, Fischer JE (1978) Vasoative intestinal peptide and the watery diarrhea syndrome. Ann Surg 187(4):411–416
Varricchi G, Galdiero MR, Loffredo S, Lucarini V, Marone G, Mattei F, Marone G, Schiavoni G (2018) Eosinophils: the unsung heroes in cancer? Oncoimmunology 7(2):e1393134
Samoszuk M (1997) Eosinophils and human cancer. Histol Histopathol 12(3):807–812
Iwasaki K, Torisu M, Fujimura T (1986) Malignant tumor and eosinophils. I. Prognostic significance in gastric cancer. Cancer 58(6):1321–1327
Davis BP, Rothenberg ME (2014) Eosinophils and cancer. Cancer Immunol Res 2(1):1–8
Acknowledgements
We thank Gulshan Singh and Loula Burton from the Department of Global Environmental Health Sciences and Office of Research Proposal Development, Tulane University for helping with figure preparation and manuscript editing, respectively.
Funding
The authors acknowledge partial financial support of NIH grant R01 AI080581 (AM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
This article is a contribution to the Special issue on: Eosinophils - Guest Editor: Hans-Uwe Simon
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Manohar, M., Kandikattu, H.K., Upparahalli Venkateshaiah, S. et al. Eosinophils in the pathogenesis of pancreatic disorders. Semin Immunopathol 43, 411–422 (2021). https://doi.org/10.1007/s00281-021-00853-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-021-00853-0