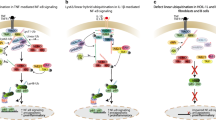
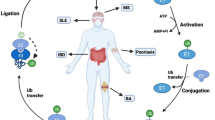
Abstract
During innate immune responses, proteostasis is greatly impacted by synthesis of pathogen proteins as well as by inflammatory tissue damage through radicals or other damaging molecules released by phagocytes. An adequate adaptation of cellular clearance pathways to the increased burden of damaged proteins is thus of fundamental importance for cells and tissues to prevent protein aggregation, inclusion body formation, and ultimately cell death. We here review the current understanding of the pivotal role of the ubiquitin proteasome system (UPS) in this proteostasis network. The proteolytic capacity of the UPS can be adjusted by differential gene expression, the incorporation and maturation kinetics of alternative active sites, and the attachment of different regulators. Dysregulation of this fine-tuning is likely to induce cell death but seen more often to promote inflammation as well. The link between proteostasis impairment and inflammation may play a crucial role in autoinflammation as well as in age-related diseases and currently uncharacterized diseases. Recent studies on proteasome-associated autoinflammatory syndromes (PRAAS) discovered that IFN signaling drives the inflammation caused by reduction of degradation capacity. Elucidation of these syndromes will reveal further insights in the understanding of inadequate immune responses. Knowledge related to the diversity of this degradation system will raise the awareness of potential pitfalls in the molecular diagnostics of autoinflammatory syndromes and may help to identify novel drug targets.


Similar content being viewed by others
References
Liu Y, Jesus AA, Marrero B et al (2014) Activated STING in a vascular and pulmonary syndrome. N Engl J Med 371:507–18. doi:10.1056/NEJMoa1312625
Sanchez GAM, de Jesus AA, Goldbach-Mansky R (2013) Monogenic autoinflammatory diseases: disorders of amplified danger sensing and cytokine dysregulation. Rheum Dis Clin North Am 39:701–34. doi:10.1016/j.rdc.2013.08.001
Yamanaka K, Sasagawa Y, Ogura T (2012) Recent advances in p97/VCP/Cdc48 cellular functions. Biochim Biophys Acta 1823:130–7. doi:10.1016/j.bbamcr.2011.07.001
Askanas V, Engel WK (2006) Inclusion-body myositis: a myodegenerative conformational disorder associated with Abeta, protein misfolding, and proteasome inhibition. Neurology 66:S39–48. doi:10.1212/01.wnl.0000192128.13875.1e
Liu Y, Ramot Y, Torrelo A et al (2012) Mutations in proteasome subunit β type 8 cause chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature with evidence of genetic and phenotypic heterogeneity. Arthritis Rheum 64:895–907. doi:10.1002/art.33368
LaFerla FM (2010) Pathways linking Abeta and tau pathologies. Biochem Soc Trans 38:993–5. doi:10.1042/BST0380993
Sulistio YA, Heese K (2015) The ubiquitin-proteasome system and molecular chaperone deregulation in Alzheimer’s disease. Mol Neurobiol. doi:10.1007/s12035-014-9063-4
LaFerla FM, Green KN, Oddo S (2007) Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci 8:499–509. doi:10.1038/nrn2168
Joshi-Barr S, Bett C, Chiang W-C et al (2014) De novo prion aggregates trigger autophagy in skeletal muscle. J Virol 88:2071–82. doi:10.1128/JVI. 02279-13
Deriziotis P, André R, Smith DM et al (2011) Misfolded PrP impairs the UPS by interaction with the 20S proteasome and inhibition of substrate entry. EMBO J 30:3065–77. doi:10.1038/emboj.2011.224
Ebstein F, Kloetzel P-M, Krüger E, Seifert U (2012) Emerging roles of immunoproteasomes beyond MHC class I antigen processing. Cell Mol Life Sci 69:2543–58. doi:10.1007/s00018-012-0938-0
Krüger E, Kloetzel P-M (2012) Immunoproteasomes at the interface of innate and adaptive immune responses: two faces of one enzyme. Curr Opin Immunol 24:77–83. doi:10.1016/j.coi.2012.01.005
Ciechanover A (2013) Intracellular protein degradation: from a vague idea through the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Bioorg Med Chem 21:3400–10. doi:10.1016/j.bmc.2013.01.056
Groll M, Ditzel L, Löwe J et al (1997) Structure of 20S proteasome from yeast at 2.4 A resolution. Nature 386:463–71. doi:10.1038/386463a0
Vigneron N, Van den Eynde BJ (2014) Proteasome subtypes and regulators in the processing of antigenic peptides presented by class I molecules of the major histocompatibility complex. Biomolecules 4:994–1025. doi:10.3390/biom4040994
Gu ZC, Enenkel C (2014) Proteasome assembly. Cell Mol Life Sci 71:4729–45. doi:10.1007/s00018-014-1699-8
Sahara K, Kogleck L, Yashiroda H, Murata S (2014) The mechanism for molecular assembly of the proteasome. Adv Biol Regul 54:51–8. doi:10.1016/j.jbior.2013.09.010
Kniepert A, Groettrup M (2014) The unique functions of tissue-specific proteasomes. Trends Biochem Sci 39:17–24. doi:10.1016/j.tibs.2013.10.004
Fabre B, Lambour T, Garrigues L et al (2015) Deciphering preferential interactions within supramolecular protein complexes: the proteasome case. Mol Syst Biol 11:771, doi: 10.15252/msb.20145497
Kriegenburg F, Poulsen EG, Koch A et al (2011) Redox control of the ubiquitin-proteasome system: from molecular mechanisms to functional significance. Antioxid Redox Signal 15:2265–99. doi:10.1089/ars.2010.3590
Seifert U, Bialy LP, Ebstein F et al (2010) Immunoproteasomes preserve protein homeostasis upon interferon-induced oxidative stress. Cell 142:613–24. doi:10.1016/j.cell.2010.07.036
Ebstein F, Voigt A, Lange N et al (2013) Immunoproteasomes are important for proteostasis in immune responses. Cell 152:935–7. doi:10.1016/j.cell.2013.02.018
Pickering AM, Koop AL, Teoh CY et al (2010) The immunoproteasome, the 20S proteasome and the PA28αβ proteasome regulator are oxidative-stress-adaptive proteolytic complexes. Biochem J 432:585–94. doi:10.1042/BJ20100878
Fehling H, Swat W, Laplace C et al (1994) MHC class I expression in mice lacking the proteasome subunit LMP-7. Science 265(80):1234–1237. doi:10.1126/science.8066463
Opitz E, Koch A, Klingel K et al (2011) Impairment of immunoproteasome function by β5i/LMP7 subunit deficiency results in severe enterovirus myocarditis. PLoS Pathog 7:e1002233. doi:10.1371/journal.ppat.1002233
Strehl B, Joeris T, Rieger M et al (2006) Immunoproteasomes are essential for clearance of Listeria monocytogenes in nonlymphoid tissues but not for induction of bacteria-specific CD8+ T cells. J Immunol 177:6238–6244. doi:10.4049/jimmunol.177.9.6238
Ishii K, Hisaeda H, Duan X et al (2006) The involvement of immunoproteasomes in induction of MHC class I-restricted immunity targeting Toxoplasma SAG1. Microbes Infect 8:1045–53. doi:10.1016/j.micinf.2005.10.023
Hussong SA, Kapphahn RJ, Phillips SL et al (2010) Immunoproteasome deficiency alters retinal proteasome’s response to stress. J Neurochem 113:1481–90. doi:10.1111/j.1471-4159.2010.06688.x
Chou B, Hisaeda H, Shen J et al (2008) Critical contribution of immunoproteasomes in the induction of protective immunity against Trypanosoma cruzi in mice vaccinated with a plasmid encoding a CTL epitope fused to green fluorescence protein. Microbes Infect 10:241–50. doi:10.1016/j.micinf.2007.11.010
Zaiss DMW, Bekker CPJ, Gröne A et al (2011) Proteasome immunosubunits protect against the development of CD8 T cell-mediated autoimmune diseases. J Immunol 187:2302–9. doi:10.4049/jimmunol.1101003
Eleftheriadis T, Pissas G, Antoniadi G et al (2013) CD8+ T-cell auto-reactivity is dependent on the expression of the immunoproteasome subunit LMP7 in exposed to lipopolysaccharide antigen presenting cells and epithelial target cells. Autoimmunity 46:439–45. doi:10.3109/08916934.2013.801460
Krause S, Kuckelkorn U, Dörner T et al (2006) Immunoproteasome subunit LMP2 expression is deregulated in Sjogren’s syndrome but not in other autoimmune disorders. Ann Rheum Dis 65:1021–7. doi:10.1136/ard.2005.045930
Hayashi T, Faustman D (2000) Defective function of the proteasome in autoimmunity: involvement of impaired NF-kappaB activation. Diabetes Technol Ther 2:415–28
Arima K, Kinoshita A, Mishima H et al (2011) Proteasome assembly defect due to a proteasome subunit beta type 8 (PSMB8) mutation causes the autoinflammatory disorder, Nakajo-Nishimura syndrome. Proc Natl Acad Sci U S A 108:14914–9. doi:10.1073/pnas.1106015108
Agarwal AK, Xing C, DeMartino GN et al (2010) PSMB8 encoding the β5i proteasome subunit is mutated in joint contractures, muscle atrophy, microcytic anemia, and panniculitis-induced lipodystrophy syndrome. Am J Hum Genet 87:866–72. doi:10.1016/j.ajhg.2010.10.031
Kitamura A, Maekawa Y, Uehara H et al (2011) A mutation in the immunoproteasome subunit PSMB8 causes autoinflammation and lipodystrophy in humans. J Clin Invest 121:4150–60. doi:10.1172/JCI58414DS1
McDermott A, Jesus AA, Liu Y et al (2013) A case of proteasome-associated auto-inflammatory syndrome with compound heterozygous mutations. J Am Acad Dermatol 69:e29–e32
Mégarbané A, Sanders A, Chouery E et al (2002) An unknown autoinflammatory syndrome associated with short stature and dysmorphic features in a young boy. J Rheumatol 29:1084–7
Torrelo A, Patel S, Colmenero I et al (2010) Chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome. J Am Acad Dermatol 62:489–95. doi:10.1016/j.jaad.2009.04.046
McDermott A, Jacks J, Kessler M et al (2014) Proteasome-associated autoinflammatory syndromes: advances in pathogeneses, clinical presentations, diagnosis, and management. Int J Dermatol. doi:10.1111/ijd.12695
Kluk J, Rustin M, Brogan PA et al (2013) CANDLE syndrome: a report of a novel mutation and review of the literature. Br J Dermatol. doi:10.1111/bjd.12600
Oyanagi K, Sasaki K, Ohama E et al (1987) An autopsy case of a syndrome with muscular atrophy, decreased subcutaneous fat, skin eruption and hyper gamma-globulinemia: peculiar vascular changes and muscle fiber degeneration. Acta Neuropathol 73:313–9
Qureshi N, Perera P-Y, Shen J et al (2003) The proteasome as a lipopolysaccharide-binding protein in macrophages: differential effects of proteasome inhibition on lipopolysaccharide-induced signaling events. J Immunol 171:1515–1525. doi:10.4049/jimmunol.171.3.1515
Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat Immunol 11:373–84. doi:10.1038/ni.1863
Katsuyama M (2010) NOX/NADPH oxidase, the superoxide-generating enzyme: its transcriptional regulation and physiological roles. J Pharmacol Sci 114:134–46
Yewdell JW (2011) DRiPs solidify: progress in understanding endogenous MHC class I antigen processing. Trends Immunol 32:548–58. doi:10.1016/j.it.2011.08.001
Warnatsch A, Bergann T, Krüger E (2013) Oxidation matters: the ubiquitin proteasome system connects innate immune mechanisms with MHC class I antigen presentation. Mol Immunol 55:106–9. doi:10.1016/j.molimm.2012.10.007
Szeto J, Kaniuk NA, Canadien V et al (2014) ALIS are stress-induced protein storage compartments for substrates of the proteasome and autophagy. Autophagy 2:189–199. doi:10.4161/auto.2731
Senft D, Ronai ZA (2015) UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem Sci. doi:10.1016/j.tibs.2015.01.002
Urano F, Wang X, Bertolotti A et al (2000) Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287:664–6
Menu P, Mayor A, Zhou R et al (2012) ER stress activates the NLRP3 inflammasome via an UPR-independent pathway. Cell Death Dis 3:e261. doi:10.1038/cddis.2011.132
Lerner AG, Upton J-P, Praveen PVK et al (2012) IRE1α induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab 16:250–64. doi:10.1016/j.cmet.2012.07.007
Kanazawa N (2012) Nakajo-Nishimura syndrome: an autoinflammatory disorder showing pernio-like rashes and progressive partial lipodystrophy. Allergol Int 61:197–206. doi:10.2332/allergolint.11-RAI-0416
Zhang X, Bogunovic D, Payelle-Brogard B et al (2014) Human intracellular ISG15 prevents interferon-α/β over-amplification and auto-inflammation. Nature 517:89–93. doi:10.1038/nature13801
Baechler EC, Batliwalla FM, Karypis G et al (2003) Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A 100:2610–5. doi:10.1073/pnas.0337679100
Crow YJ (2013) Aicardi-Goutières syndrome. Handb Clin Neurol 113:1629–35. doi:10.1016/B978-0-444-59565-2.00031-9
Briggs TA, Rice GI, Daly S et al (2011) Tartrate-resistant acid phosphatase deficiency causes a bone dysplasia with autoimmunity and a type I interferon expression signature. Nat Genet 43:127–31. doi:10.1038/ng.748
Qian M-X, Pang Y, Liu CH et al (2013) Acetylation-mediated proteasomal degradation of core histones during DNA repair and spermatogenesis. Cell 153:1012–24. doi:10.1016/j.cell.2013.04.032
Stadtmueller BM, Kish-Trier E, Ferrell K et al (2012) Structure of a proteasome Pba1-Pba2 complex: implications for proteasome assembly, activation, and biological function. J Biol Chem 287:37371–82. doi:10.1074/jbc.M112.367003
Fricke B, Heink S, Steffen J et al (2007) The proteasome maturation protein POMP facilitates major steps of 20S proteasome formation at the endoplasmic reticulum. EMBO Rep 8:1170–5. doi:10.1038/sj.embor.7401091
Hirano Y, Hendil KB, Yashiroda H et al (2005) A heterodimeric complex that promotes the assembly of mammalian 20S proteasomes. Nature 437:1381–5. doi:10.1038/nature04106
Paraskevopoulos K, Kriegenburg F, Tatham MH et al (2014) Dss1 is a 26S proteasome ubiquitin receptor. Mol Cell 56:453–61. doi:10.1016/j.molcel.2014.09.008
Kim Y-C, DeMartino GN (2011) C termini of proteasomal ATPases play nonequivalent roles in cellular assembly of mammalian 26S proteasome. J Biol Chem 286:26652–66. doi:10.1074/jbc.M111.246793
Cascio P (2014) PA28αβ: the enigmatic magic ring of the proteasome? Biomolecules 4:566–84. doi:10.3390/biom4020566
De Graaf N, van Helden MJG, Textoris-Taube K et al (2011) PA28 and the proteasome immunosubunits play a central and independent role in the production of MHC class I-binding peptides in vivo. Eur J Immunol 41:926–35. doi:10.1002/eji.201041040
Mao I, Liu J, Li X, Luo H (2008) REGgamma, a proteasome activator and beyond? Cell Mol Life Sci 65:3971–80. doi:10.1007/s00018-008-8291-z
Lehmann A, Niewienda A, Jechow K et al (2010) Ecm29 fulfils quality control functions in proteasome assembly. Mol Cell 38:879–88. doi:10.1016/j.molcel.2010.06.016
Gorbea C, Goellner GM, Teter K et al (2004) Characterization of mammalian Ecm29, a 26S proteasome-associated protein that localizes to the nucleus and membrane vesicles. J Biol Chem 279:54849–61. doi:10.1074/jbc.M410444200
Tomko RJ Jr, Hochstrasser M (2013) Molecular architecture and assembly of the eukaryotic proteasome. Annu Rev Biochem. doi:10.1146/annurev-biochem-060410-150257
Acknowledgments
The authors thank R. Goldbach-Mansky for great collaboratory work and for reviewing the manuscript and K. E. Gilling for critical reading of the manuscript. This research was supported by the Deutsche Forschungsgemeinschaft SFB740 and SFB TR 43 to EK.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is a contribution to the Special Issue on The Inflammasome and Autoinflammatory Diseases - Guest Editors: Seth L. Masters, Tilmann Kallinich and Seza Ozen
Rights and permissions
About this article
Cite this article
Brehm, A., Krüger, E. Dysfunction in protein clearance by the proteasome: impact on autoinflammatory diseases. Semin Immunopathol 37, 323–333 (2015). https://doi.org/10.1007/s00281-015-0486-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-015-0486-4