Abstract
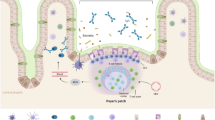
Under normal and pathological conditions, lymphocyte migration into the gastrointestinal mucosa to form gut-associated lymphoid tissue is mediated by the L-selectin ligand peripheral lymph node addressin and the integrin α4β7 ligand mucosal addressin cell adhesion molecule 1 (MAdCAM-1) expressed on high endothelial venules (HEVs) and HEV-like vessels. In this review, we discuss these two distinct lymphocyte homing systems involved in the pathogenesis of chronic inflammatory gastrointestinal diseases with reference to our and others’ previously published works. We also describe a recently developed recombinant integrin α4β7 heterodimeric IgG chimera that can be used as an immunohistochemical reagent to stain functional MAdCAM-1.










Similar content being viewed by others
References
Corr SC, Gahan CC, Hill C (2008) M-cells: origin, morphology and role in mucosal immunity and microbial pathogenesis. FEMS Immunol Med Microbiol 52:2–12
Butcher EC, Picker LJ (1996) Lymphocyte homing and homeostasis. Science 572:60–66
von Andrian UH, Mempel TR (2003) Homing and cellular traffic in lymph nodes. Nat Rev Immunol 3:867–878
Rosen SD (2004) Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol 22:129–156
Streeter PR, Rouse BT, Butcher EC (1988) Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol 107:1853–1862
Yeh JC, Hiraoka N, Petryniak B, Nakayama J, Ellies LG, Rabuka D, Hindsgaul O, Marth JD, Lowe JB, Fukuda M (2001) Novel sulfated lymphocyte homing receptors and their control by a core 1 extension β1,3-N-acetylglucosaminyltransferase. Cell 105:957–969
Hemmerich S, Leffler H, Rosen SD (1995) Structure of the O-glycans in GlyCAM-1, an endothelial-derived ligand for L-selectin. J Biol Chem 270:12035–12047
Streeter PR, Berg EL, Rouse BT, Bargatze RF, Butcher EC (1988) A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature 331:41–46
Bargatze RF, Jutila MA, Butcher EC (1995) Distinct roles of L-selectin and integrins α4β7 and LAF-1 in lymphocyte homing to Peyer’s patch-HEV in situ: the multistep model confirmed and refined. Immunity 3:99–108
Berlin C, Bargatze RF, Campbell JJ, von Andrian UH, Szabo MC, Hasslen SR, Nelson RD, Berg EL, Erlandsen SL, Butcher EC (1995) α4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell 80:413–422
Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, Weissman IL, Hamann A, Butcher EC (1993) α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74:185–195
Berg EL, McEvoy LM, Berlin C, Bargatze RF, Butcher EC (1993) L-selectin-mediated lymphocyte rolling on MAdCAM-1. Nature 366:695–698
Aloisi F, Pujol-Borrell R (2006) Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol 6:205–217
Renkonen J, Tynninen O, Hayry P, Paavonen T, Renkonen R (2002) Glycosylation might provide endothelial zip codes for organ-specific leukocyte traffic into inflammatory sites. Am J Pathol 161:543–550
van Dinther-Janssen AC, Pals ST, Scheper R, Breedveld F, Meijer CJ (1990) Dendritic cells and high endothelial venules in the rheumatoid synovial membrane. J Rheumatol 17:11–17
Kabel PJ, Voorbij HA, de Haan-Meulman M, Pals ST, Drexhage HA (1989) High endothelial venules present in lymphoid cell accumulations in thyroids affected by autoimmune disease: a study in men and BB rats of functional activity and development. J Clin Endocrinol Metab 68:744–751
Dogan A, Du M, Koulis A, Briskin MJ, Isaacson PG (1997) Expression of lymphocyte homing receptors and vascular addressins in low-grade gastric B-cell lymphomas of mucosa-associated lymphoid tissue. Am J Pathol 151:1361–1369
Kobayashi M, Mitoma J, Nakamura N, Katsuyama T, Nakayama J, Fukuda M (2004) Induction of peripheral lymph node addressin in human gastric mucosa infected by Helicobacter pylori. Proc Natl Acad Sci U S A 101:17807–17812
Salmi M, Granfors K, MacDermott R, Jalkanen S (1994) Aberrant binding of lamina propria lymphocytes to vascular endothelium in inflammatory bowel diseases. Gastroenterology 106:596–605
Suzawa K, Kobayashi M, Sakai Y, Hoshino H, Watanabe M, Harada O, Ohtani H, Fukuda M, Nakayama J (2007) Preferential induction of peripheral lymph node addressin on high endothelial venule-like vessels in the active phase of ulcerative colitis. Am J Gastroenterol 102:1499–1509
Kobayashi M, Hoshino H, Masumoto J, Fukushima M, Suzawa K, Kageyama S, Suzuki M, Ohtani H, Fukuda M, Nakayama J (2009) GlcNAc6ST-1-mediated decoration of MAdCAM-1 protein with L-selectin ligand carbohydrates directs disease activity of ulcerative colitis. Inflamm Bowel Dis 15:697–706
Peek RM Jr, Blaser MJ (2002) Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer 2:28–37
Hidaka E, Ota H, Hidaka H, Hayama M, Matsuzawa K, Akamatsu T, Nakayama J, Katsuyama T (2001) Helicobacter pylori and two ultrastructurally distinct layers of gastric mucous cell mucins in the surface of mucous gel layer. Gut 49:474–480
Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG (1991) Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 338:1175–1176
Yuasa Y (2003) Control of gut differentiation and intestinal-type gastric carcinogenesis. Nat Rev Cancer 3:592–600
Nomura A, Stemmermann GN, Chyou PH, Kato I, Perez-Perez GI, Blaser MJ (1991) Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med 325:1132–1136
Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK (1991) Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med 325:1127–1131
Higashi H, Tsutsumi R, Muto S, Sugiyama T, Azuma T, Asaka M, Hatakeyama M (2002) SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science 295:683–686
Duijvestijn AM, Horst E, Pals ST, Rouse BN, Steere AC, Picker LJ, Meijer CJ, Butcher EC (1988) High endothelial differentiation in human lymphoid and inflammatory tissues defined by monoclonal antibody HECA-452. Am J Pathol 130:147–155
Kumamoto K, Mitsuoka C, Izawa M, Kimura N, Otsubo N, Ishida H, Kiso M, Yamada T, Hirohashi S, Kannagi R (1998) Specific detection of sialyl Lewis X determinant carried on the mucin GlcNAcβ1 → 6GalNAcα core structure as a tumor-associated antigen. Biochem Biophys Res Commun 247:514–517
Kobayashi M, Mitoma J, Hoshino H, Yu SY, Shimojo Y, Suzawa K, Khoo KH, Fukuda M, Nakayama J (2011) Prominent expression of sialyl Lewis X-capped core 2-branched O-glycans on high endothelial venules in gastric MALT lymphoma. J Pathol 224:67–77
Dixon MF, Genta RM, Yardley JH, Correa P (1996) Classification and grading of gastritis. The updated Sydney system. Am J Surg Pathol 20:1161–1181
Pablos JL, Santiago B, Tsay D, Singer MS, Palao G, Galindo M, Rosen SD (2005) A HEV-restricted sulfotransferase is expressed in rheumatoid arthritis synovium and is induced by lymphotoxin-α/β and TNF-α in cultured endothelial cells. BMC Immunol 6:6
Drayton DL, Ying X, Lee J, Lesslauer W, Ruddle NH (2003) Ectopic LTαβ directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J Exp Med 197:1153–1163
Weninger W, Carlsen HS, Goodarzi MF, Crowley MA, Baekkevold ES, Cavanagh LL, von Andrian UH (2003) Naive T cell recruitment to nonlymphoid tissues: a role for endothelium-expressed CC chemokine ligand 21 in autoimmune disease and lymphoid neogenesis. J Immunol 170:4638–4648
Manzo A, Bugatti S, Caporali R, Prevo R, Jackson DG, Uguccioni M, Buckley CD, Montecucco C, Pitzalis C (2007) CCL21 expression pattern of human secondary lymphoid organ stroma is conserved in inflammatory lesions with lymphoid neogenesis. Am J Pathol 171:1549–1562
Ohara H, Isomoto H, Wen CY, Ejima C, Murata M, Miyazaki M, Takeshima F, Mizuta Y, Murata I, Koji T, Nagura H, Kohno S (2003) Expression of mucosal addressin cell adhesion molecule 1 on vascular endothelium of gastric mucosa in patients with nodular gastritis. World J Gastroenterol 9:2701–2705
Feagan BG, Greenberg GR, Wild G, Fedorak RN, Pare P, McDonald JW, Dube R, Cohen A, Steinhart AH, Landau S, Aguzzi RA, Fox IH, Vandervoort MK (2005) Treatment of ulcerative colitis with a humanized antibody to the α4β7 integrin. N Engl J Med 352:2499–2507
Hussell T, Isaacson PG, Crabtree JE, Spencer J (1993) The response of cells from low-grade B-cell gastric lymphomas of mucosa-associated lymphoid tissue to Helicobacter pylori. Lancet 342:571–574
Hussell T, Isaacson PG, Crabtree JE, Spencer J (1996) Helicobacter pylori-specific tumour-infiltrating T cells provide contact dependent help for the growth of malignant B cells in low-grade gastric lymphoma of mucosa-associated lymphoid tissue. J Pathol 178:122–127
Isaacson PG, Du MQ (2004) MALT lymphoma: from morphology to molecules. Nat Rev Cancer 4:644–653
Salmi M, Jalkanen S (1998) Endothelial ligands and homing of mucosal leukocytes in extraintestinal manifestations of IBD. Inflamm Bowel Dis 4:149–156
Schroeder KW, Tremaine WJ, Ilstrup DM (1987) Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 317:1625–1629
Bibiloni R, Fedorak RN, Tannock GW, Madsen KL, Gionchetti P, Campieri M, De Simone C, Sartor RB (2005) VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol 100:1539–1546
Briskin M, Winsor-Hines D, Shyjan A, Cochran N, Bloom S, Wilson J, McEvoy LM, Butcher EC, Kassam N, Mackay CR, Newman W, Ringler DJ (1997) Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol 151:97–110
Souza HS, Elia CC, Spencer J, MacDonald TT (1999) Expression of lymphocyte–endothelial receptor-ligand pairs, α4β7/MAdCAM-1 and OX40/OX40 ligand in the colon and jejunum of patients with inflammatory bowel disease. Gut 45:856–863
Arihiro S, Ohtani H, Suzuki M, Murata M, Ejima C, Oki M, Kinouchi Y, Fukushima K, Sasaki I, Nakamura S, Matsumoto T, Torii A, Toda G, Nagura H (2002) Differential expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in ulcerative colitis and Crohn’s disease. Pathol Int 52:367–374
Uchimura K, Muramatsu H, Kaname T, Ogawa H, Yamakawa T, Fan QW, Mitsuoka C, Kannagi R, Habuchi O, Yokoyama I, Yamamura K, Ozaki T, Nakagawara A, Kadomatsu K, Muramatsu T (1998) Human N-acetylglucosamine-6-O-sulfotransferase involved in the biosynthesis of 6-sulfo sialyl Lewis X: molecular cloning, chromosomal mapping, and expression in various organs and tumor cells. J Biochem 124:670–678
Li X, Tedder TF (1999) CHST1 and CHST2 sulfotransferases expressed by human vascular endothelial cells: cDNA cloning, expression, and chromosomal localization. Genomics 55:345–347
Bistrup A, Bhakta S, Lee JK, Belov YY, Gunn MD, Zuo FR, Huang CC, Kannagi R, Rosen SD, Hemmerich S (1999) Sulfotransferases of two specificities function in the reconstitution of high endothelial cell ligands for L-selectin. J Cell Biol 145:899–910
Hiraoka N, Petryniak B, Nakayama J, Tsuboi S, Suzuki M, Yeh JC, Tanaka T, Miyasaka M, Lowe JB, Fukuda M (1999) A novel, high endothelial venule-specific sulfotransferase expresses 6-sulfo sialyl Lewis X, an L-selectin ligand displayed by CD34. Immunity 11:79–89
Kawashima H, Petryniak B, Hiraoka N, Mitoma J, Huckaby V, Nakayama J, Uchimura K, Kadomatsu K, Muramatsu T, Lowe JB, Fukuda M (2005) N-acetylglucosamine-6-O-sulfotransferase 1 and 2 cooperatively control lymphocyte homing through L-selectin ligand biosynthesis in high endothelial venules. Nat Immunol 6:1096–1104
Uchimura K, Gauguet JM, Singer MS, Tsay D, Kannagi R, Muramatsu T, von Andrian UH, Rosen SD (2005) A major class of L-selectin ligands is eliminated in mice deficient in two sulfotransferases expressed in high endothelial venules. Nat Immunol 6:1105–1113
Arata-Kawai H, Singer MS, Bistrup A, Zante A, Wang YQ, Ito Y, Bao X, Hemmerich S, Fukuda M, Rosen SD (2011) Functional contributions of N- and O-glycans to L-selectin ligands in murine and human lymphoid organs. Am J Pathol 178:423–433
Hirakawa J, Tsuboi K, Sato K, Kobayashi M, Watanabe S, Takakura A, Imai Y, Ito Y, Fukuda M, Kawashima H (2010) Novel anti-carbohydrate antibodies reveal the cooperative function of sulfated N- and O-glycans in lymphocyte homing. J Biol Chem 285:40864–40878
Mitoma J, Bao X, Petryniak B, Schaerli P, Gauguet JM, Yu SY, Kawashima H, Saito H, Ohtsubo K, Marth JD, Khoo KH, von Andrian UH, Lowe JB, Fukuda M (2007) Critical functions of N-glycans in L-selectin-mediated lymphocyte homing and recruitment. Nat Immunol 8:409–418
Gordon FH, Hamilton MI, Donoghue S, Greenlees C, Palmer T, Rowley-Jones D, Dhillon AP, Amlot PL, Pounder RE (2002) A pilot study of treatment of active ulcerative colitis with natalizumab, a humanized monoclonal antibody to α4 integrin. Aliment Pharmacol Ther 16:699–705
Ghosh S, Goldin E, Gordon FH, Malchow HA, Rask-Madsen J, Rutgeerts P, Vyhnalek P, Zadorova Z, Palmer T, Donoghue S (2003) Natalizumab for active Crohn’s disease. N Engl J Med 348:24–32
Soler D, Chapman T, Yang LL, Wyant T, Egan R, Fedyk ER (2009) The binding specificity and selective antagonism of vedolizumab, and anti-α4β7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther 330:864–875
Rutgeerts P, Vermeire S, Van Assche G (2009) Biological therapies for inflammatory bowel diseases. Gastroenterology 136:1182–1197
Aarden L, Ruuls SR, Wolbink G (2008) Immunogenicity of anti-tumor necrosis factor antibodies: toward improved methods of anti-antibody measurement. Curr Opin Immunol 20:431–435
Nechansky A (2010) HAHA, nothing to laugh about: measuring the immunogenicity (human anti-human antibody response) induced by humanized monoclonal antibodies applying ELISA and SPR technology. J Pharm Biomed Anal 51:252–254
Hoshino H, Kobayashi M, Mitoma J, Sato Y, Fukuda M, Nakayama J (2011) An integrin α4β7⋅IgG heterodimeric chimera binds to MAdCAM-1 on high endothelial venules in gut-associated lymphoid tissue. J Histochem Cytochem 59:572–583
Stephens PE, Ortlepp S, Perkins VC, Robinson MK, Kirby H (2000) Expression of a soluble functional form of the integrin α4β1 in mammalian cells. Cell Adhes Commun 7:377–390
Stamper HB Jr, Woodruff JJ (1976) Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J Exp Med 144:828–833
Acknowledgments
We thank the members of the Fukudas’ and Nakayama’s laboratories for their critical contribution to the studies and useful discussion. We also thank Dr. Elise Lamar for critical reading of the manuscript. The work reviewed from our laboratories has been supported by Grants-in-Aid for Young Scientist B-18790240, B-20790278, and B-22790343 (to M.K.), for Scientific Research on Priority Area 14082201 (to J.N) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Grant-in-Aid for Scientific Research B-18390113 and B-21390104 from the Japan Society for the Promotion of Science (to J.N.), and Grants RO1 CA48737 and CA33000 and PO1 CA71932 from the National Institutes of Health (to M.F.).
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published as part of the Special Issue on Glycosylation and Immunity [34:3].
Rights and permissions
About this article
Cite this article
Kobayashi, M., Hoshino, H., Suzawa, K. et al. Two distinct lymphocyte homing systems involved in the pathogenesis of chronic inflammatory gastrointestinal diseases. Semin Immunopathol 34, 401–413 (2012). https://doi.org/10.1007/s00281-012-0302-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-012-0302-3