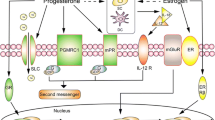
Abstract
In different species of mammal, uterine natural killer (uNK) cells are massively recruited and presented at the fetal maternal interface with a spatio-temporal pattern, and regarded as a constructive element to support reproductive development. Recent insights highlight the uNK cells activation, function and interaction with local compartments, which all contribute to the initiation of vascular structural changes. New trends of uNK cells research will benefit the diagnosis, management and test treatment strategy of preeclampsia. Furthermore, we suggest that more efforts and specific studies are needed to further explore the role of uNK cells at the unique micro-environment.


Similar content being viewed by others
References
Lanier LL (2006) Natural killer cells: roundup. Immunol Rev 214:5–8
Seaman WE (2000) Natural killer cells and natural killer T cells. Arthritis Rheum 43:1204–1217
King A (2000) Uterine leukocytes and decidualization. Hum Reprod Updat 6:28–36
Peel S (1989) Granulated metrial gland cells. Adv Anat Embryol Cell Biol 115:1–112
Anne Croy B, van den Heuvel MJ, Borzychowski AM, Tayade C (2006) Uterine natural killer cells: a specialized differentiation regulated by ovarian hormones. Immunol Rev 214:161–185
Moffett-King A (2002) Natural killer cells and pregnancy. Nat Rev Immunol 2:656–663
Head JR (1996) Uterine natural killer cells during pregnancy in rodents. Nat Immun 15:7–21
Zhang J, Croy BA, Tian Z (2005) Uterine natural killer cells: their choices, their missions. Cell Mol Immunol 2:123–129
Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F, Masch R, Lockwood CJ, Schachter AD, Park PJ, Strominger JL (2003) Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med 198:1201–1212
Cooper MA, Fehniger TA, Caligiuri MA (2001) The biology of human natural killer-cell subsets. Trends Immunol 22:633–640
Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA (2001) Human natural killer cells: a unique innate immunoregulatory role for the CD56bright subset. Blood 97:3146–3151
Kopcow HD, Allan DSJ, Chen X, Rybalov B, Andzelm MM, Ge B, Strominger JL (2005) Human decidual NK cells form immature activating synapses and are not cytotoxic. PNAS 102:15563–15568
Heuvel MJ, Xie X, Tayade C, Peralta C, Fang Y, Leonard S, Paffaro VA, Sheikhi AK, Murrant C, Anne Croy B (2005) A review of trafficking and activation of uterine natural killer cells. Am J Reprod Immunol 54:322–331
van den Heuvel M, Peralta C, Bashar S, Taylor S, Horrocks J, Croy BA (2005) Trafficking of peripheral blood CD56(bright) cells to the decidualizing uterus—new tricks for old dogmas? J Reprod Immunol 67:21–34
Herington JL, Bany BM (2007) Effect of the Conceptus on uterine natural killer cell numbers and function in the mouse uterus during decidualization. Biol Reprod 76:579–588
King A, Burrows T, Loke YW (1996) Human uterine natural killer cells. Nat Immun 15:41–52
Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, Benharroch D, Porgador A, Keshet E, Yagel S, Mandelboim O (2006) Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 12:1065–1074
Leonard S, Murrant C, Tayade C, van den Heuvel M, Watering R, Croy BA (2006) Mechanisms regulating immune cell contributions to spiral artery modification—facts and hypotheses—a review. Placenta 27(Suppl A):S40–S46
Ashkar AA, Croy BA (2001) Functions of uterine natural killer cells are mediated by interferon gamma production during murine pregnancy. Semin Immunol 13:235–241
Eriksson M, Meadows SK, Basu S, Mselle TF, Wira CR, Sentman CL (2006) TLRs Mediate IFN-{gamma} Production by Human Uterine NK Cells in Endometrium. J Immunol 176:6219–6224
Sargent IL, Borzychowski AM, Redman CWG (2006) NK cells and human pregnancy—an inflammatory view. Trends Immunol 27:399–404
Moffett A, Regan L, Braude P (2004) Natural killer cells, miscarriage, and infertility. BMJ 329:1283–1285
Tabiasco J, Rabot M, Aguerre-Girr M, El Costa H, Berrebi A, Parant O, Laskarin G, Juretic K, Bensussan A, Rukavina D, Le Bouteiller P (2006) Human decidual NK cells: unique phenotype and functional properties—a review. Placenta 27(Suppl A):S34–S39
Le Bouteiller P, Tabiasco J (2006) Killers become builders during pregnancy. Nat Med 12:991–992
Pijnenborg R, Vercruysse L, Hanssens M (2006) The uterine spiral arteries in human pregnancy: facts and controversies. Placenta 27:939–958
Sibai B, Dekker G, Kupferminc M (2005) Pre-eclampsia. Lancet 365:785–799
Dekker GA, Sibai BM (1998) Etiology and pathogenesis of preeclampsia: current concepts. Am J Obstet Gynecol 179:1359–1375
Noris M, Perico N, Remuzzi G (2005) Mechanisms of disease: pre-eclampsia. Nat Clin Pract Nephrol 1:98–114 (quiz 120)
Chaouat G, Ledee-bataille N, Zourbas S, Dubanchet S, Sandra O, Martal J, Ostojojic S, Frydman R (2003) Implantation: can immunological parameters of implantation failure be of interest for pre-eclampsia? J Reprod Immunol 59:205–217
Moffett A, Loke C (2006) Immunology of placentation in eutherian mammals. Nat Rev Immunol 6:584–594
Craven CM, Morgan T, Ward K (1998) Decidual spiral artery remodelling begins before cellular interaction with cytotrophoblasts. Placenta 19:241–252
Aplin JD (1991) Implantation, trophoblast differentiation and haemochorial placentation: mechanistic evidence in vivo and in vitro. J Cell Sci 99:681–692
Berthold H, Louis LHP (2005) Vascular biology in implantation and placentation. Angiogenesis V8:157–167
Redman CW, Sargent IL (2005) Latest advances in understanding preeclampsia. Science 308:1592–1594
Parham P (2004) NK cells and trophoblasts: partners in pregnancy. J Exp Med 200:951–955
Slukvin, II, Breburda EE, Golos TG (2004) Dynamic changes in primate endometrial leukocyte populations: differential distribution of macrophages and natural killer cells at the rhesus monkey implantation site and in early pregnancy. Placenta 25:297–307
Trundley A, Moffett A (2004) Human uterine leukocytes and pregnancy. Tissue Antigens 63:1–12
Hirano H, Imai Y, Ito H (2002) Spiral artery of placenta: development and pathology-immunohistochemical, microscopical, and electron-microscopic study. Kobe J Med Sci 48:13–23
Adamson SL, Lu Y, Whiteley KJ, Holmyard D, Hemberger M, Pfarrer C, Cross JC (2002) Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev Biol 250:358–373
Croy BA, He H, Esadeg S, Wei Q, McCartney D, Zhang J, Borzychowski A, Ashkar AA, Black GP, Evans SS, Chantakru S, van den Heuvel M, Paffaro VA Jr, Yamada AT (2003) Uterine natural killer cells: insights into their cellular and molecular biology from mouse modelling. Reproduction 126:149–160
Cross JC, Hemberger M, Lu Y, Nozaki T, Whiteley K, Masutani M, Adamson SL (2002) Trophoblast functions, angiogenesis and remodeling of the maternal vasculature in the placenta. Mol Cell Endocrinol 187:207–212
Kanellopoulos-Langevin C, Caucheteux SM, Verbeke P, Ojcius DM (2003) Tolerance of the fetus by the maternal immune system: role of inflammatory mediators at the feto-maternal interface. Reprod Biol Endocrinol 1:121
Georgiades P, Ferguson-Smith AC, Burton GJ (2002) Comparative developmental anatomy of the murine and human definitive placentae. Placenta 23:3–19
Croy BA, Esadeg S, Chantakru S, van den Heuvel M, Paffaro VA, He H, Black GP, Ashkar AA, Kiso Y, Zhang J (2003) Update on pathways regulating the activation of uterine Natural Killer cells, their interactions with decidual spiral arteries and homing of their precursors to the uterus. J Reprod Immunol 59:175–191
Chantakru S, Kuziel WA, Maeda N, Croy BA (2001) A study on the density and distribution of uterine Natural Killer cells at mid pregnancy in mice genetically-ablated for CCR2, CCR 5 and the CCR5 receptor ligand, MIP-1 alpha. J Reprod Immunol 49:33–47
Esadeg S, He H, Pijnenborg R, Van Leuven F, Croy BA (2003) Alpha-2 macroglobulin controls trophoblast positioning in mouse implantation sites. Placenta 24:912–921
Naicker T, Khedun SM, Moodley J, Pijnenborg R (2003) Quantitative analysis of trophoblast invasion in preeclampsia. Acta Obstet Gynecol Scand 82:722–729
Ain R, Canham LN, Soares MJ (2003) Gestation stage-dependent intrauterine trophoblast cell invasion in the rat and mouse: novel endocrine phenotype and regulation. Dev Biol 260:176–190
Guimond MJ, Wang B, Fujita J, Terhorst C, Croy BA (1996) Pregnancy-associated uterine granulated metrial gland cells in mutant and transgenic mice. Am J Reprod Immunol 35:501–509
Barber EM, Pollard JW (2003) The uterine NK cell population requires IL-15 but these cells are not required for pregnancy nor the resolution of a Listeria monocytogenes infection. J Immunol 171:37–46
Ashkar AA, Croy BA (1999) Interferon-gamma contributes to the normalcy of murine pregnancy. Biol Reprod 61:493–502
Croy BA, Ashkar AA, Foster RA, DiSanto JP, Magram J, Carson D, Gendler SJ, Grusby MJ, Wagner N, Muller W, Guimond MJ (1997) Histological studies of gene-ablated mice support important functional roles for natural killer cells in the uterus during pregnancy. J Reprod Immunol 35:111–133
Guimond MJ, Luross JA, Wang B, Terhorst C, Danial S, Croy BA (1997) Absence of natural killer cells during murine pregnancy is associated with reproductive compromise in TgE26 mice. Biol Reprod 56:169–179
Greenwood JD, Minhas K, di Santo JP, Makita M, Kiso Y, Croy BA (2000) Ultrastructural studies of implantation sites from mice deficient in uterine natural killer cells. Placenta 21:693–702
Croy BA, Chantakru S, Esadeg S, Ashkar AA, Wei Q (2002) Decidual natural killer cells: key regulators of placental development (a review). J Reprod Immunol 57:151–168
Guimond MJ, Wang B, Croy BA (1998) Engraftment of bone marrow from severe combined immunodeficient (SCID) mice reverses the reproductive deficits in natural killer cell-deficient tg epsilon 26 mice. J Exp Med 187:217–223
King A, Burrows T, Verma S, Hiby S, Loke YW (1998) Human uterine lymphocytes. Hum Reprod Updat 4:480–485
Arcuri F, Cintorino M, Carducci A, Papa S, Riparbelli MG, Mangioni S, Di Blasio AM, Tosi P, Vigano P (2006) Human decidual natural killer cells as a source and target of macrophage migration inhibitory factor. Reproduction 131:175–182
Lanier LL (2005) NK cell recognition. Annu Rev Immunol 23:225–274
Chaouat G, Cayol V, Mairovitz V, Dubanchet S (1999) Localization of the Th2 cytokines IL-3, IL-4, IL-10 at the fetomaternal interface during human and murine pregnancy and lack of requirement for Fas/Fas ligand interaction for a successful allogeneic pregnancy. Am J Reprod Immunol 42:1–13
van den Heuvel MJ, Xie X, Tayade C, Peralta C, Fang Y, Leonard S, Paffaro VA Jr, Sheikhi AK, Murrant C, Croy BA (2005) A review of trafficking and activation of uterine natural killer cells. Am J Reprod Immunol 54:322–331
Murphy SP, Fast LD, Hanna NN, Sharma S (2005) Uterine NK Cells Mediate Inflammation-Induced Fetal Demise in IL-10-Null Mice. J Immunol 175:4084–4090
Zenclussen AC (2006) Regulatory T cells in pregnancy. Springer Semin Immunopathol 28:31–39
Tang Q, Bluestone JA (2006) Plasmacytoid DCs and Treg cells: casual acquaintance or monogamous relationship? Nat Immunol 7:551–553
Abrahams VM, Mor G (2005) Toll-like receptors and their role in the trophoblast. Placenta 26:540–547
Mor G, Romero R, Aldo PB, Abrahams VM (2005) Is the trophoblast an immune regulator? The role of Toll-like receptors during pregnancy. Crit Rev Immunol 25:375–388
Zhang JH, He H, Borzychowski AM, Takeda K, Akira S, Croy BA (2003) Analysis of cytokine regulators inducing interferon production by mouse uterine natural killer cells. Biol Reprod 69:404–411
Xie X, Kang Z, Anderson LN, He H, Lu B, Anne Croy B (2005) Analysis of the contributions of L-selectin and CXCR3 in mediating leukocyte homing to pregnant mouse uterus. Am J Reprod Immunol 53:1–12
Xie X, He H, Colonna M, Seya T, Takai T, Croy BA (2005) Pathways participating in activation of mouse uterine natural killer cells during pregnancy. Biol Reprod 73:510–518
Ashkar AA, Black GP, Wei Q, He H, Liang L, Head JR, Croy BA (2003) Assessment of requirements for IL-15 and IFN regulatory factors in uterine NK cell differentiation and function during pregnancy. J Immunol 171:2937–2944
Ashkar AA, Di Santo JP, Croy BA (2000) Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med 192:259–270
Bachmayer N, Rafik Hamad R, Liszka L, Bremme K, Sverremark-Ekstrom E (2006) Aberrant uterine natural killer (NK)-cell expression and altered placental and serum levels of the NK-cell promoting cytokine interleukin-12 in pre-eclampsia. Am J Reprod Immunol 56:292–301
Kastelein RA, Hunter CA, Cua DJ (2007) Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol 25
Iwakura Y, Ishigame H (2006) The IL-23/IL-17 axis in inflammation. J Clin Invest 116:1218–1222
Groothuis PG, Nap AW, Winterhager E, Grummer R (2005) Vascular development in endometriosis. Angiogenesis V8:147–156
van der Meer A, Lukassen HGM, van Cranenbroek B, Weiss EH, Braat DDM, van Lierop MJ, Joosten I (2006) Soluble HLA-G promotes Th1-type cytokine production by cytokine-activated uterine and peripheral natural killer cells. Mol Hum Reprod 13:123–133
Frankenstein Z, Alon U, Cohen IR (2006) The immune-body cytokine network defines a social architecture of cell interactions. Biol Direct 1:32
Goswami D, Tannetta DS, Magee LA, Fuchisawa A, Redman CWG, Sargent IL, von Dadelszen P (2006) Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta 27:56–61
Parra M, Rodrigo R, Barja P, Bosco C, Fernandez V, Munoz H, Soto-Chacon E (2005) Screening test for preeclampsia through assessment of uteroplacental blood flow and biochemical markers of oxidative stress and endothelial dysfunction. Am J Obstet Gynecol 193:1486–1491
Eriksson M, Meadows SK, Wira CR, Sentman CL (2006) Endogenous transforming growth factor-beta inhibits toll-like receptor mediated activation of human uterine natural killer cells. Am J Reprod Immunol 56:321–328
Jianhong Zhang, Haiming Wei, Dongmei Wu, Tian Z (2006) Toll-like receptor 3 agonist induces impairment of uterine vascular remodeling and fetal losses in CBA×DBA/2 mice. J Reprod Immunol (in press). DOI 10.1016/j.jri.2006.10.005
Ligam P, Manuelpillai U, Wallace EM, Walker D (2005) Localisation of indoleamine 2,3-dioxygenase and kynurenine hydroxylase in the human placenta and decidua: implications for role of the kynurenine pathway in pregnancy. Placenta 26:498–504
Wu X, Jin LP, Yuan MM, Zhu Y, Wang MY, Li DJ (2005) Human first-trimester trophoblast cells recruit CD56brightCD16- NK cells into decidua by way of expressing and secreting of CXCL12/stromal cell-derived factor 1. J Immunol 175:61–68
Bulmer JN, Lash GE (2005) Human uterine natural killer cells: a reappraisal. Mol Immunol 42:511–521
Lash GE, Schiessl B, Kirkley M, Innes BA, Cooper A, Searle RF, Robson SC, Bulmer JN (2006) Expression of angiogenic growth factors by uterine natural killer cells during early pregnancy. J Leukoc Biol 80:572–580
Li XF, Charnock-Jones DS, Zhang E, Hiby S, Malik S, Day K, Licence D, Bowen JM, Gardner L, King A, Loke YW, Smith SK (2001) Angiogenic growth factor messenger ribonucleic acids in uterine natural killer cells. J Clin Endocrinol Metab 86:1823–1834
Hiby SE, Walker JJ, O’Shaughnessy K M, Redman CW, Carrington M, Trowsdale J, Moffett A (2004) Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med 200:957–965
King A, Hiby SE, Gardner L, Joseph S, Bowen JM, Verma S, Burrows TD, Loke YW (2000) Recognition of trophoblast HLA class I molecules by decidual NK cell receptors—a review. Placenta 21(Suppl A):S81–S85
Chaouat G, Ledee-Bataille N, Dubanchet S (2005) Immunological similarities between implantation and pre-eclampsia. Am J Reprod Immunol 53:222–229
Le Bouteiller P, Pizzato N, Barakonyi A, Solier C (2003) HLA-G, pre-eclampsia, immunity and vascular events. J Reprod Immunol 59:219–234
Yie S-m, Li L-h, Li Y-m, Librach C (2004) HLA-G protein concentrations in maternal serum and placental tissue are decreased in preeclampsia. Am J Obstet Gynecol 191:525–529
Jones RL, Stoikos C, Findlay JK, Salamonsen LA (2006) TGF-{beta} superfamily expression and actions in the endometrium and placenta. Reproduction 132:217–232
Huppertz B, Kadyrov M, Kingdom JCP (2006) Apoptosis and its role in the trophoblast. Am J Obstet Gynecol 195:29–39
Matthiesen L, Berg G, Ernerudh J, Ekerfelt C, Jonsson Y, Sharma S (2005) Immunology of preeclampsia. Chem Immunol Allergy 89:49–61
Kadyrov M, Kingdom JCP, Huppertz B (2006) Divergent trophoblast invasion and apoptosis in placental bed spiral arteries from pregnancies complicated by maternal anemia and early-onset preeclampsia/intrauterine growth restriction. Am J Obstet Gynecol 194:557–563
Lash GE, Otun HA, Innes BA, Kirkley M, De Oliveira L, Searle RF, Robson SC, Bulmer JN (2006) Interferon-{gamma} inhibits extravillous trophoblast cell invasion by a mechanism that involves both changes in apoptosis and protease levels. FASEB J 20:2512–2518
Hu Y, Dutz JP, MacCalman CD, Yong P, Tan R, von Dadelszen P (2006) Decidual NK cells alter in vitro first trimester extravillous cytotrophoblast migration: a role for IFN–{gamma}. J Immunol 177:8522–8530
Gammill HS, Lin C, Hubel CA (2007) Endothelial progenitor cells and preeclampsia. Front Biosci 12:2383–2394
Wei J, Satomi M, Negishi Y, Matsumura Y, Miura A, Nishi Y, Asakura H, Takeshita T (2006) Effect of sera on the adhesion of natural killer cells to the endothelium in severe pre-eclampsia. J Obstet Gynaecol Res 32:443–448
Matsubara K, Nagamatsu T, Fujii T, Kozuma S, Taketani Y (2005) Lymphokine-activated killer cells induced from decidual lymphocytes reduce the angiogenic activity of trophoblasts by enhancing the release of soluble fms-like tyrosine kinase-1 from trophoblasts: an implication for the pathophysiology of preeclampsia. J Reprod Immunol 68:27–37
Levine RJ, Maynard SE, Qian C, Lim K-H, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA (2004) Circulating Angiogenic Factors and the Risk of Preeclampsia. N Engl J Med 350:672–683
Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA (2003) Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111:649–658
Luttun A, Carmeliet P (2003) Soluble VEGF receptor Flt1: the elusive preeclampsia factor discovered? J Clin Invest 111:600–602
Tjoa ML, Levine RJ, Karumanchi SA (2007) Angiogenic factors and preeclampsia. Front Biosci 12:2395–2402
Crocker IP, Wareing M, Ferris GR, Jones CJ, Cartwright JE, Baker PN, Aplin JD (2005) The effect of vascular origin, oxygen, and tumour necrosis factor alpha on trophoblast invasion of maternal arteries in vitro. J Pathol 206:476–485
Bryceson YT, March ME, Ljunggren H-G, Long EO (2006) Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev 214:73–91
Gasser S, Raulet DH (2006) Activation and self-tolerance of natural killer cells. Immunol Rev 214:130–142
Caucheteux SM, Kanellopoulos-Langevin C, Ojcius DM (2003) At the innate frontiers between mother and fetus: linking abortion with complement activation. Immunity 18:169–172
Varla-Leftherioti M, Spyropoulou-Vlachou M, Niokou D, Keramitsoglou T, Darlamitsou A, Tsekoura C, Papadimitropoulos M, Lepage V, Balafoutas C, Stavropoulos-Giokas C (2003) Natural killer (NK) cell receptors’ repertoire in couples with recurrent spontaneous abortions. Am J Reprod Immunol 49:183–191
Dosiou C, Giudice LC (2005) Natural killer cells in pregnancy and recurrent pregnancy loss: endocrine and immunologic perspectives. Endocr Rev 26:44–62
van den Heuvel MJ, Horrocks J, Bashar S, Taylor S, Burke S, Hatta K, Lewis JE, Croy BA (2005) Menstrual cycle hormones induce changes in functional interactions between lymphocytes and decidual vascular endothelial cells. J Clin Endocrinol Metab 90:2835–2842
Chantakru S, Wang WC, van den Heuvel M, Bashar S, Simpson A, Chen Q, Croy BA, Evans SS (2003) Coordinate regulation of lymphocyte-endothelial interactions by pregnancy-associated hormones. J Immunol 171:4011–4019
Huang ST, Vo KC, Lyell DJ, Faessen GH, Tulac S, Tibshirani R, Giaccia AJ, Giudice LC (2004) Developmental response to hypoxia. FASEB J 18:1348–1365
Greer IA (2005) Pre-eclampsia matters. BMJ 330:549–550
Wagner LK (2004) Diagnosis and management of preeclampsia. Am Fam Physician 70:2317–2324
Sibai BM (2005) Diagnosis, prevention, and management of eclampsia. Obstet Gynecol 105:402–410
Borzychowski AM, Croy BA, Chan WL, Redman CW, Sargent IL (2005) Changes in systemic type 1 and type 2 immunity in normal pregnancy and pre-eclampsia may be mediated by natural killer cells. Eur J Immunol 35:3054–3063
Henderson TA, Saunders PT, Moffett-King A, Groome NP, Critchley HO (2003) Steroid receptor expression in uterine natural killer cells. J Clin Endocrinol Metab 88:440–449
Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, Rotmensch S, Romero R (2003) Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol 189:1063–1069
Yagel S, Goldman-Wohl DS, Mandelboim O, Hanna Y, Hochner-Celnikier D (2005) Maternal adaptation to vasculogenesis and angiogenesis at the feto-maternal interface. Thromb Res 115(Suppl 1):97–99
Carmeliet P (2005) Angiogenesis in life, disease and medicine. Nature 438:932–936
Saftlas AF, Beydoun H, Triche E (2005) Immunogenetic determinants of preeclampsia and related pregnancy disorders: a systematic review. Obstet Gynecol 106:162–172
Sargent IL, Borzychowski AM, Redman CW (2006) Immunoregulation in normal pregnancy and pre–eclampsia: an overview. Reprod Biomed Online 13:680–686
Zhang C, Zhang J, Tian Z (2006) The regulatory effect of natural killer cells: do “NK-reg cells” exist? Cell Mol Immunol 3:241–254
Blouw B, Song H, Tihan T, Bosze J, Ferrara N, Gerber H-P, Johnson RS, Bergers G (2003) The hypoxic response of tumors is dependent on their microenvironment. Cancer Cell 4:133–146
Helige C, Hagendorfer G, Smolle J, Dohr G (2001) Uterine natural killer cells in a three–dimensional tissue culture model to study trophoblast invasion. Lab Invest 81:1153–1162
Zenclussen AC, Fest S, Joachim R, Klapp BF, Arck PC (2004) Introducing a mouse model for pre-eclampsia: adoptive transfer of activated Th1 cells leads to pre-eclampsia-like symptoms exclusively in pregnant mice. Eur J Immunol 34:377–387
Wulff C, Weigand M, Kreienberg R, Fraser HM (2003) Angiogenesis during primate placentation in health and disease. Reproduction 126:569–577
Acknowledgment
This work is supported by the Natural Science Foundation of China (No. 30630059, 30528007, 30570819, 30571695 and 30500467) and Ministry of Science & Technology of China (973 Basic Science Project nos. 2006CB504300 and #2006CB806504).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, J., Tian, Z. UNK cells: their role in tissue re-modelling and preeclampsia. Semin Immunopathol 29, 123–133 (2007). https://doi.org/10.1007/s00281-007-0068-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-007-0068-1