Abstract.
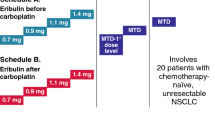
Purpose: To determine the maximum tolerated dose (MTD) of irinotecan combined with carboplatin, to evaluate its efficacy and toxicity for patients with lung cancer, and to examine its pharmacokinetics and pharmacodynamics. Methods: The dose of irinotecan was escalated from 40 mg/m2 per week in increments of 10 mg/m2. Carboplatin was fixed at 300 mg/m2. Multivariate regression models with an interaction term were used to evaluate synergistic pharmacodynamic interactions. Results: The MTD and recommended dose of irinotecan were 60 and 50 mg/m2, respectively. Dose-limiting toxicities were grade 4 neutropenia and grade 3 or 4 diarrhea. In phase II studies, response rates were 81.3% (95% confidence interval 61.8–100%) in 16 patients with small-cell lung cancer and 22.2% (2.7–41.8%) in 18 patients with non-small-cell lung cancer. Two patients (6%) experienced grade 4 neutropenia, thrombocytopenia, and grade 3 diarrhea. The area under the plasma concentration versus time curve (AUC) of carboplatin ranged from 2.87 to 9.31 mg·min/ml, with a median of 4.66 mg·min/ml. In pharmacodynamic analyses, the log-transformed surviving fraction in platelet count (SFp) showed a significant association with the AUC of carboplatin (P=0.010), while that in neutrophil count (SFn) was not significantly correlated with any pharmacokinetic parameter. The interaction term was not significant in either case. Conclusions: These results indicate that AUC-based dosing of carboplatin is still rational in combination chemotherapy. A more sensitive method for predicting life-threatening toxicities is needed, however, because traditional pharmacokinetic parameters were not adequate tools for identifying patients at high risk of severe neutropenia and diarrhea. This combination regimen has only modest activity, and further studies are necessary to evaluate a different dose schedule.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Sato, M., Ando, M., Minami, H. et al. Phase I/II and pharmacologic study of irinotecan and carboplatin for patients with lung cancer. Cancer Chemother Pharmacol 48, 481–487 (2001). https://doi.org/10.1007/s002800100355
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s002800100355