Abstract.
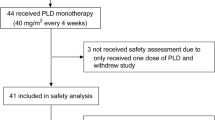
Purpose: A phase II study was designed to assess the efficacy and safety of Caelyx (liposomal doxorubicin) in patients with advanced or metastatic gastric cancer. Methods: A total of 25 patients with gastric adenocarcinoma were treated with Caelyx 45 mg/m2 every 28 days as first-line therapy for advanced disease. Patients were treated until tumour progression or unacceptable toxicity. Results: One patient was withdrawn from the study after experiencing a severe infusion reaction. Of the 24 evaluable patients, 1 had a partial response, 7 had stable disease and the others progressed. Side effects, in particular palmar-plantar erythrodysaesthesia and haematological toxicity, were minor. Conclusions: We conclude that while this dose and schedule of Caelyx in this patient group is acceptable, further studies with this regimen cannot be recommended due to the lack of antitumour activity seen.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Thomas, A.L., O'Byrne, K., Furber, L. et al. A phase II study of Caelyx, liposomal doxorubicin: lack of activity in patients with advanced gastric cancer. Cancer Chemother Pharmacol 48, 266–268 (2001). https://doi.org/10.1007/s002800100351
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s002800100351