Abstract
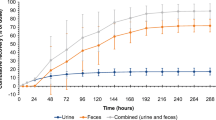
Raltitrexed (Tomudex) is a specific inhibitor of thymidylate synthase and has recently been licensed in Europe for use in the treatment of advanced colorectal carcinoma. This study evaluated the metabolism, excretion and pharmacokinetics after a single dose of 3.0 mg/m2 [14C]-raltitrexed in patients with advanced solid malignancies not amenable to curative therapy. From April 1994 to July 1995, nine patients (six men and three women) were recruited into the study. Pharmacokinetics analysis was performed during the first cycle of treatment in all patients and, in two patients, limited sampling was done prior to and during the second cycle of treatment. The mean observed peak plasma concentration (Cmax) was 700.6 ng/ml and the median time (t max) to reach maximal raltitrexed concentrations was 15 min after the initiation of the infusion. After reaching Cmax the drug declined in a triexponential manner with a terminal half-life of 257 h. The AUC0–∞ as predicted by the pharmacokinetic model was 2341.7 ng h ml−1. Clearance was 41.3 ml/min, of which renal clearance accounted for 50–60%. Urinary collection for the measurement of radiolabeled drug revealed that renal excretion extrapolated to infinity accounted for 40% of the total radioactive dose. Faecal excretion accounted for only 3% of the dose when samples were collected to day 5 in the first six patients. Collection was extended to 10 days in the last three patients and faecal elimination accounted for 14% in these patients. Raltitrexed measurements prior to subsequent doses suggest that there was no accumulation of the drug with repeated administration. Low levels of radioactivity measured in the red cell pellets on days 15, 22 and 29 are likely to represent drug retained by newly forming red cells at the time of dosing. Examination of the urine revealed that the drug was excreted unchanged. The toxicities seen were in line with those encountered in previous studies. Grade 3 and 4 thrombocytopenia occurred in three patients and grade 3 neutropenia occurred in two patients.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 29 July 1997 / Accepted: 23 October 1997
Rights and permissions
About this article
Cite this article
Beale, P., Judson, I., Hanwell, J. et al. Metabolism, excretion and pharmacokinetics of a single dose of [14C]-raltitrexed in cancer patients. Cancer Chemother Pharmacol 42, 71–76 (1998). https://doi.org/10.1007/s002800050787
Issue Date:
DOI: https://doi.org/10.1007/s002800050787