Abstract
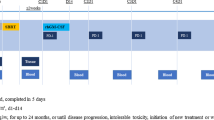
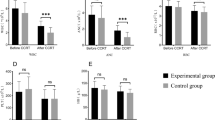
From June 1991 to August 1994, 61 patients with stage III unresectable non-small-cell lung cancer (NSCLC; 16 cases of stage IIIA with N2 bulky disease and 45 cases of stage IIIB) were treated with ifosfamide given i.v. at 3 g/m2 on day 1, carboplatin given i.v. at 200 mg/m2 on days 1 and 2, etoposide given i.v. at 120 mg/m2 on days 1–3 (ICE) and recombinant human granulocyte colony-stimulating factor (rhG-CSF) given s.c. at 5 μg/kg on days 4–13. Chemotherapy was given every 3 weeks for up to three cycles and, unless the disease progressed, was followed by thoracic radiotherapy on the tumor volume (total dose 60 Gy) and mediastinum (40 Gy). All patients had measurable or evaluable unresectable disease and a performance status (Eastern Cooperative Oncology Group) of 0–1. Only 61% of the enrolled patients received the full program of chemoradiotherapy according to the study design. At the end of sequential chemo-radiothera-peutic treatment, 41% of the patients had an objective response (24 partial responses and 1 complete response), 31% showed no change and 28% had progressive disease. The response rate noted for patients in stage IIIA with N2 bulky disease and that recorded for patients in stage IIIB did not differ significantly. The median time to progression was 5.4 months and the median survival was 8.2 months, with the 1-year survival rate being 31%. Sites of progression were mostly intrathoracic. Haematological toxicity was the main side effect, with grade III–IV thrombocytopenia being reported in 24% of the 165 courses of intensive ICE chemotherapy given. Febrile neutropenia was described in six courses (three patients). Non-haematological toxicities and radiotherapy-related side effects were generally mild and easily manageable. In conclusion, in unresectable stage III NSCLC a short program of moderately intensified ICE chemotherapy with rhG-CSF protection followed by sequential radiotherapy failed to increase the percentage of objective responses and reached a median survival comparable with that previously achieved with standard doses.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 26 November 1995/Accepted: 5 March 1996
Rights and permissions
About this article
Cite this article
Scagliotti, G., Ricardi, U., Crinó, L. et al. Phase II study of intensive chemotherapy with carboplatin, ifosfamide and etoposide plus recombinant human granulocyte colony-stimulating factor and sequential radiotherapy in locally advanced, unresectable non-small-cell lung cancer. Cancer Chemother Pharmacol 38, 561–565 (1996). https://doi.org/10.1007/s002800050527
Issue Date:
DOI: https://doi.org/10.1007/s002800050527