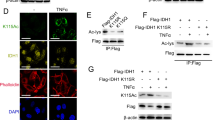
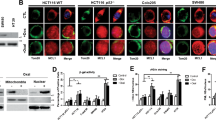
Abstract
Purpose
Enhancing chemotherapy sensitivity in colorectal cancer (CRC) is critical for improving treatment outcomes. TMEM120A has been reported to interact with coenzyme A (CoA), but its biological significance in CRC is unknown. In this study, we aimed to investigate the functional implications of TMEM120A in CRC and its impact on chemotherapy sensitivity.
Methods
Stable knockout of TMEM120A in CRC cell lines was conducted using CRISPR/Cas9 technology. Overexpression of various derivatives of TMEM120A was achieved through lentiviral transduction. Cell fractionation was employed to isolate the nuclear and cytoplasmic fraction. Total histones were isolated by acid extraction and then subjected to determine histone acetylation levels using western blot analysis. Cell viability was evaluated using the MTS assay.
Results
We demonstrate that TMEM120A’s nuclear localization is crucial for its role in regulating CRC chemosensitivity. Mechanistically, the nuclear subpopulation of TMEM120A plays a key role in sustaining the nuclear CoA levels, which in turn influences the levels of nuclear acetyl-CoA and histone acetylation in CRC cells. Notably, direct inhibition of histone acetylation recapitulated the phenotypic effects observed upon TMEM120A depletion, leading to increased chemosensitivity in CRC cells.
Conclusion
Our study provides novel insights into the role of TMEM120A in modulating chemotherapy sensitivity in CRC. Nuclear TMEM120A regulates CoA levels, which in turn modulates nuclear acetyl-CoA levels and histone acetylation, thereby influencing the response of CRC cells to chemotherapy agents. Targeting TMEM120A-mediated pathways may represent a promising strategy for enhancing chemotherapy efficacy in CRC treatment.







Similar content being viewed by others
Data availability
Data generated or analyzed during this study are available from the corresponding author upon reasonable request.
References
Hossain MS et al (2022) Colorectal cancer: a review of carcinogenesis, global epidemiology, current challenges, risk factors, preventive and treatment strategies. Cancers 14:1732. https://doi.org/10.3390/cancers14071732
Biller LH, Schrag D (2021) Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA 325:669–685. https://doi.org/10.1001/jama.2021.0106
McQuade RM, Stojanovska V, Bornstein JC, Nurgali K (2017) Colorectal cancer chemotherapy: the evolution of treatment and new approaches. Curr Med Chem 24:1537–1557. https://doi.org/10.2174/0929867324666170111152436
Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B (2017) The different mechanisms of cancer drug resistance: a brief review. Adv Pharm Bull 7:339–348. https://doi.org/10.15171/apb.2017.041
Alfarouk KO et al (2015) Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp. Cancer Cell Int 15:71. https://doi.org/10.1186/s12935-015-0221-1
Bradshaw PC (2021) Acetyl-CoA metabolism and histone acetylation in the regulation of aging and lifespan. Antioxidants (Basel, Switzerland) 10:572. https://doi.org/10.3390/antiox10040572
Yi CH, Vakifahmetoglu-Norberg H, Yuan J (2011) Integration of apoptosis and metabolism. Cold Spring Harb Symp Quant Biol 76:375–387. https://doi.org/10.1101/sqb.2011.76.010777
Martínez-Reyes I, Chandel NS (2018) Acetyl-CoA-directed gene transcription in cancer cells. Genes Dev 32:463–465. https://doi.org/10.1101/gad.315168.118
Feron O (2019) The many metabolic sources of acetyl-CoA to support histone acetylation and influence cancer progression. Ann Transl Med 7:S277. https://doi.org/10.21037/atm.2019.11.140
Sebastián C, Mostoslavsky R (2017) The various metabolic sources of histone acetylation. Trends Endocrinol Metab 28:85–87. https://doi.org/10.1016/j.tem.2016.11.001
Jung G, Hernández-Illán E, Moreira L, Balaguer F, Goel A (2020) Epigenetics of colorectal cancer: biomarker and therapeutic potential. Nat Rev Gastroenterol Hepatol 17:111–130. https://doi.org/10.1038/s41575-019-0230-y
Qin J, Wen B, Liang Y, Yu W, Li H (2020) Histone modifications and their role in colorectal cancer (review). Pathol Oncol Res POR 26:2023–2033. https://doi.org/10.1007/s12253-019-00663-8
Nenkov M, Ma Y, Gaßler N, Chen Y (2021) Metabolic reprogramming of colorectal cancer cells and the microenvironment: implication for therapy. Int J Mol Sci 22:6262. https://doi.org/10.3390/ijms22126262
Beaulieu-Laroche L et al (2020) TACAN is an ion channel involved in sensing mechanical pain. Cell 180:956-967.e917. https://doi.org/10.1016/j.cell.2020.01.033
Batrakou DG, de Las Heras JI, Czapiewski R, Mouras R, Schirmer EC (2015) TMEM120A and B: nuclear envelope transmembrane proteins important for adipocyte differentiation. PLoS One 10:e0127712. https://doi.org/10.1371/journal.pone.0127712
Li S et al (2022) Gain-of-function genetic screening identifies the antiviral function of TMEM120A via STING activation. Nat Commun 13:105. https://doi.org/10.1038/s41467-021-27670-1
Rong Y et al (2021) TMEM120A contains a specific coenzyme A-binding site and might not mediate poking- or stretch-induced channel activities in cells. Elife 10:e71474. https://doi.org/10.7554/eLife.71474
Xue J et al (2021) TMEM120A is a coenzyme A-binding membrane protein with structural similarities to ELOVL fatty acid elongase. Elife 10:e71220. https://doi.org/10.7554/eLife.71220
Guertin DA, Wellen KE (2023) Acetyl-CoA metabolism in cancer. Nat Rev Cancer 23:156–172. https://doi.org/10.1038/s41568-022-00543-5
St Paul M et al (2021) Coenzyme A fuels T cell anti-tumor immunity. Cell Metab 33:2415-2427.e2416. https://doi.org/10.1016/j.cmet.2021.11.010
He W, Li Q, Li X (2023) Acetyl-CoA regulates lipid metabolism and histone acetylation modification in cancer. Biochim Biophys Acta Rev Cancer 1878:188837. https://doi.org/10.1016/j.bbcan.2022.188837
Ran FA et al (2013) Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8:2281–2308. https://doi.org/10.1038/nprot.2013.143
Shechter D, Dormann HL, Allis CD, Hake SB (2007) Extraction, purification and analysis of histones. Nat Protoc 2:1445–1457. https://doi.org/10.1038/nprot.2007.202
Gout I (2018) Coenzyme A, protein CoAlation and redox regulation in mammalian cells. Biochem Soc Trans 46:721–728. https://doi.org/10.1042/bst20170506
Pietrocola F, Galluzzi L, Bravo-San Pedro JM, Madeo F, Kroemer G (2015) Acetyl coenzyme A: a central metabolite and second messenger. Cell Metab 21:805–821. https://doi.org/10.1016/j.cmet.2015.05.014
Srinivasan B, Sibon OC (2014) Coenzyme A, more than ‘just’ a metabolic cofactor. Biochem Soc Trans 42:1075–1079. https://doi.org/10.1042/bst20140125
Zhang B, Chen D, Liu B, Dekker FJ, Quax WJ (2020) A novel histone acetyltransferase inhibitor A485 improves sensitivity of non-small-cell lung carcinoma cells to TRAIL. Biochem Pharmacol 175:113914. https://doi.org/10.1016/j.bcp.2020.113914
Lasko LM et al (2017) Discovery of a selective catalytic p300/CBP inhibitor that targets lineage-specific tumours. Nature 550:128–132. https://doi.org/10.1038/nature24028
Wang N, Ma T, Yu B (2023) Targeting epigenetic regulators to overcome drug resistance in cancers. Signal Transduct Target Ther 8:69. https://doi.org/10.1038/s41392-023-01341-7
Wu D, Qiu Y, Jiao Y, Qiu Z, Liu D (2020) Small molecules targeting HATs, HDACs, and BRDs in cancer therapy. Front Oncol 10:560487. https://doi.org/10.3389/fonc.2020.560487
Peserico A, Simone C (2011) Physical and functional HAT/HDAC interplay regulates protein acetylation balance. J Biomed Biotechnol 2011:371832. https://doi.org/10.1155/2011/371832
Bishop TR et al (2023) Acetyl-CoA biosynthesis drives resistance to histone acetyltransferase inhibition. Nat Chem Biol. https://doi.org/10.1038/s41589-023-01320-7
Hogg SJ et al (2021) Targeting histone acetylation dynamics and oncogenic transcription by catalytic P300/CBP inhibition. Mol Cell 81:2183-2200.e2113. https://doi.org/10.1016/j.molcel.2021.04.015
Lee JH, Choy ML, Marks PA (2012) Mechanisms of resistance to histone deacetylase inhibitors. Adv Cancer Res 116:39–86. https://doi.org/10.1016/b978-0-12-394387-3.00002-1
Robey RW et al (2011) Histone deacetylase inhibitors: emerging mechanisms of resistance. Mol Pharm 8:2021–2031. https://doi.org/10.1021/mp200329f
Funding
We would like to clarify that this research project was conducted without any external funding or financial support. The study was carried out as part of our academic research efforts and was not supported by any specific grants or funding sources.
Author information
Authors and Affiliations
Contributions
Conceptualization: LW and XL; Resources: XL; Data curation: XL; Formal analysis: LW and XL; Supervision: XL; Validation: LW and XL; Investigation: LW and XL; Visualization: XL; Methodology: LW and XL; Project administration: XL; Writing—original draft: LW; Writing—review and editing: LW and XL.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, L., Liu, X. TMEM120A-mediated regulation of chemotherapy sensitivity in colorectal cancer cells. Cancer Chemother Pharmacol 93, 11–22 (2024). https://doi.org/10.1007/s00280-023-04594-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-023-04594-9