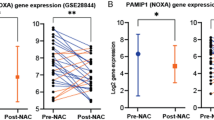
Abstract
Purpose
Despite the beneficial effects of chemotherapy, therapy-induced senescence (TIS) manifests itself as an undesirable byproduct. Preclinical evidence suggests that tumor cells undergoing TIS can re-emerge as more aggressive divergents and contribute to recurrence, and thus, senolytics were proposed as adjuvant treatment to eliminate senescent tumor cells. However, the identification of TIS in clinical samples is essential for the optimal use of senolytics in cancer therapy. In this study, we aimed to detect and quantify TIS using matched breast cancer samples collected pre- and post-exposure to neoadjuvant chemotherapy (NAC).
Methods
Detection of TIS was based on the change in gene and protein expression levels of three senescence-associated markers (downregulation of Lamin B1 and Ki-67 and upregulation of p16INK4a).
Results
Our analysis revealed that 23 of 72 (31%) of tumors had a shift in the protein expression of the three markers after exposure to NAC suggestive of TIS. Gene expression sets of two independent NAC-treated breast cancer samples showed consistent changes in the expression levels of LMNB1, MKI67 and CDKN2A.
Conclusions
Collectively, our study shows a more individualized approach to measure TIS hallmarks in matched breast cancer samples and provides an estimation of the extent of TIS in breast cancer clinically. Results from this work should be complemented with more comprehensive identification approaches of TIS in clinical samples in order to adopt a more careful implementation of senolytics in cancer treatment.





Similar content being viewed by others
Data availability
The patients’ datasets generated during the current work are not publicly available due to patients’ privacy concerns of the institutional review board policies on human tissue data but are available upon request from the corresponding author. The data of the gene expression profiles that were utilized in support the findings of this work are available from the Gene Expression Omnibus (GEO) database (reference numbers: GSE28844 https://doi.org/10.1371/journal.pone.0053983 and GSE21974 https://doi.org/10.3892/or.2011.1392).
References
Saleh T, Bloukh S, Carpenter VJ, Alwohoush E, Bakeer J, Darwish S, Azab B, Gewirtz DA (2020) Therapy-induced senescence: an “old” friend becomes the enemy. Cancers (Basel) 12:822. https://doi.org/10.3390/cancers12040822
Wang B, Kohli J, Demaria M (2020) Senescent cells in cancer therapy: friends or foes? Trends Cancer 6:838–857. https://doi.org/10.1016/j.trecan.2020.05.004
Beck J, Horikawa I, Harris C (2020) Cellular senescence: mechanisms, morphology, and mouse models. Vet Pathol 57:747–757. https://doi.org/10.1177/0300985820943841
Wang X, Tsao S-W, Wong Y-C, Cheung A (2003) Induction of senescent-like growth arrest as a new target in anticancer treatment. Curr Cancer Drug Targets 3:153–159. https://doi.org/10.2174/1568009033482001
Robles SJ, Adami GR (1998) Agents that cause DNA double strand breaks lead to P16INK4a enrichment and the premature senescence of normal fibrolasts. Oncogene 16:1113–1123. https://doi.org/10.1038/sj.onc.1201862
Chang BD, Broude EV, Dokmanovic M, Zhu H, Ruth A, Xuan Y, Kandel ES, Lausch E, Christov K, Roninson IB (1999) A senescence-like phenotype distinguishes tumor cells that undergo terminal proliferation arrest after exposure to anticancer agents. Cancer Res 59:3761–3767. https://doi.org/10.1038/nrc2961
Rodier F, Muñoz DP, Teachenor R, Chu V, Le O, Bhaumik D, Coppé JP, Campeau E, Beauséjour CM, Kim SH et al (2011) DNA-SCARS: distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J Cell Sci 124:68–81. https://doi.org/10.1242/jcs.071340
Rodier F, Coppé J, Patil CK, Hoeijmakers WAM, Muñoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J (2009) Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol 11:973–979. https://doi.org/10.1038/ncb1909
Hsu CH, Altschuler SJ, Wu LF (2019) Patterns of early P21 dynamics determine proliferation-senescence cell fate after chemotherapy. Cell 178:361-373.e12. https://doi.org/10.1016/J.CELL.2019.05.041
Brenner AJ, Stampfer MR, Aldaz CM (1998) Increased P16 expression with first senescence arrest in human mammary epithelial cells and extended growth capacity with P16 inactivation. Oncogene 17:199–205. https://doi.org/10.1038/sj.onc.1201919
Saleh T, Carpenter VJ, Bloukh S, Gewirtz DA (2022) Targeting tumor cell senescence and polyploidy as potential therapeutic strategies. Semin Cancer Biol 81:37–47. https://doi.org/10.1016/J.SEMCANCER.2020.12.010
Mosieniak G, Sliwinska MA, Alster O, Strzeszewska A, Sunderland P, Piechota M, Was H, Sikora E (2015) Polyploidy formation in doxorubicin-treated cancer cells can favor escape from senescence. Neoplasia 17:882–893. https://doi.org/10.1016/j.neo.2015.11.008
Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskensi M, Rubelj I, Pereira-Smith O et al (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci 92:9363–9367. https://doi.org/10.1073/pnas.92.20.9363
Kurz DJDJ, Decary S, Hong Y, Erusalimsky JDJD (2000) Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J Cell Sci 113:3613–3622. https://doi.org/10.1242/jcs.113.20.3613
Casella G, Munk R, Kim KM, Piao Y, De S, Abdelmohsen K, Gorospe M (2019) Transcriptome signature of cellular senescence. Nucl Acids Res 47:7294–7305. https://doi.org/10.1093/nar/gkz555
Hernandez-Segura A, de Jong TV, Melov S, Guryev V, Campisi J, Demaria M (2017) Unmasking transcriptional heterogeneity in senescent cells. Curr Biol 27:2652–2660. https://doi.org/10.1016/j.cub.2017.07.033
Schleich K, Kase J, Dörr JR, Trescher S, Bhattacharya A, Yu Y, Wailes EM, Fan DNY, Lohneis P, Milanovic M et al (2020) H3K9me3-mediated epigenetic regulation of senescence in mice predicts outcome of lymphoma patients. Nat Commun. https://doi.org/10.1038/s41467-020-17467-z
Davalos AR, Coppe JP, Campisi J, Desprez PY (2010) Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev 29:273–283. https://doi.org/10.1007/s10555-010-9220-9
Saleh T, Tyutynuk-Massey L, Cudjoe EKEK, Idowu MOMO, Landry JWJW, Gewirtz DADA, Tyutyunyk-Massey L, Cudjoe EKEK, Idowu MOMO, Landry JWJW et al (2018) Non-cell autonomous effects of the senescence-associated secretory phenotype in cancer therapy. Front Oncol 8:1–14. https://doi.org/10.3389/fonc.2018.00164
Sikora E, Mosieniak G, Alicja Sliwinska M (2016) Morphological and functional characteristic of senescent cancer cells. Curr Drug Targets 17:377–387. https://doi.org/10.2174/1389450116666151019094724
Elmore LW, Rehder CW, Di X, McChesney PA, Jackson-cook CK, Gewirtz DA, Holt SE (2002) Adriamycin-induced senescence in breast tumor cells involves functional P53 and telomere dysfunction. J Biol Chem 277:35509–35515. https://doi.org/10.1074/jbc.M205477200
Patel NH, Bloukh S, Alwohosh E, Alhesa A, Saleh T, Gewirtz DA (2021) Autophagy and senescence in cancer therapy. Adv Cancer Res 150:1–74. https://doi.org/10.1016/BS.ACR.2021.01.002
Zhang JW, Zhang SS, Song JR, Sun K, Zong C, Zhao QD, Liu WT, Li R, Wu MC, Wei LX (2014) Autophagy inhibition switches low-dose camptothecin-induced premature senescence to apoptosis in human colorectal cancer cells. Biochem Pharmacol 90:265–275. https://doi.org/10.1016/j.bcp.2014.05.009
Goehe RW, Di X, Sharma K, Bristol ML, Henderson SC, Valerie K, Rodier F, Davalos AR, Gewirtz DA (2012) The autophagy-senescence connection in chemotherapy: must tumor cells (self) eat before they sleep? J Pharmacol Exp Ther 343:763–778
Saleh T, Tyutyunyk-Massey L, Murray GFGF, Alotaibi MRMRMR, Kawale ASASAS, Elsayed Z, Henderson SCSC, Yakovlev V, Elmore LWLWLW, Toor A et al (2019) Tumor cell escape from therapy-induced senescence. Biochem Pharmacol 162:202–212. https://doi.org/10.1016/j.bcp.2018.12.013
Milanovic M, Yu Y, Schmitt CA (2018) The senescence-stemness alliance—a cancer-hijacked regeneration principle. Trends Cell Biol 28:1049–1061. https://doi.org/10.1016/j.tcb.2018.09.001
Yang L, Fang J, Chen J (2017) Tumor cell senescence response produces aggressive variants. Cell Death Discov 3:17049. https://doi.org/10.1038/cddiscovery.2017.49
Duy C, Li M, Teater M, Meydan C, Garrett-bakelman FE, Lee TC, Chin CR, Durmaz C, Kawabata KC, Dhimolea E et al (2021) Chemotherapy induces senescence-like resilient cells capable of initiating AML recurrence. Cancer Discov 11:candisc.1375.2020. https://doi.org/10.1158/2159-8290.CD-20-1375
Demaria M, Leary MNO, Chang J, Shao L, Liu S, Alimirah F, Koenig K, Le C, Mitin N, Deal AM et al (2017) Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov 7:165–177. https://doi.org/10.1158/2159-8290.CD-16-0241
Bojko A, Czarnecka-Herok J, Charzynska A, Dabrowski M, Sikora E (2019) Diversity of the senescence phenotype of cancer cells treated with chemotherapeutic agents. Cells 8:1501. https://doi.org/10.3390/cells8121501
Sharpless NE, Sherr CJ (2015) Forging a signature of in vivo senescence. Nat Rev Cancer 15:397–408. https://doi.org/10.1038/nrc3960
Chakradeo S, Elmore LW, Gewirtz DA (2016) Is senescence reversible? Curr Drug Targets 17:460–466. https://doi.org/10.2174/1389450116666150825113500
Litwiniec A, Gackowska L, Helmin-Basa A, Żuryń A, Grzanka A (2013) Low-dose etoposide-treatment induces endoreplication and cell death accompanied by cytoskeletal alterations in A549 cells: does the response involve senescence? the possible role of vimentin. Cancer Cell Int 13:9. https://doi.org/10.1186/1475-2867-13-9
Palaniyappan A (2009) Cyclophosphamide induces premature senescence in normal human fibroblasts by activating MAP kinases. Biogerontology 10:677–682. https://doi.org/10.1007/s10522-009-9215-5
Vijayaraghavan S, Karakas C, Doostan I, Chen X, Bui T, Yi M, Raghavendra AS, Zhao Y, Bashour SI, Ibrahim NK et al (2017) CDK4/6 and autophagy inhibitors synergistically induce senescence in Rb positive cytoplasmic cyclin e negative cancers. Nat Commun 8:1–17. https://doi.org/10.1038/ncomms15916
Fleury H, Malaquin N, Tu V, Gilbert S, Martinez A, Olivier MA, Sauriol A, Communal L, Leclerc-Desaulniers K, Carmona E et al (2019) Exploiting interconnected synthetic lethal interactions between PARP inhibition and cancer cell reversible senescence. Nat Commun 10:2556. https://doi.org/10.1038/s41467-019-10460-1
Ewald JA, Joshua AD, Church DR, Yang B, Hyang W, Laurila TA, Jarrard DF (2016) Androgen deprivation induces senescence characteristics in prostate cancer cells in vitro and in vivo. Prostate 73:337–345. https://doi.org/10.1002/pros.22571.Androgen
Roberson RS, Kussick SJ, Vallieres E, Chen SYJ, Wu DY (2005) Escape from therapy-induced accelerated cellular senescence in P53-null lung cancer cells and in human lung cancers. Cancer Res 65:2795–2803. https://doi.org/10.1158/0008-5472.CAN-04-1270
Cotarelo CL, Schad A, Kirkpatrick CJ, Sleeman JP, Springer E, Schmidt M, Thaler S (2016) Detection of cellular senescence within human invasive breast carcinomas distinguishes different breast tumor subtypes. Oncotarget 7:74846–748597. https://doi.org/10.18632/oncotarget.12432
Poele RH, Okorokov AL, Jardine L, Cummings J, Joel SP, te Poele RH, Okorokov AL, Jardine L, Cummings J, Joel SP (2002) DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res 62:1876–1883
Short S, Fielder E, Miwa S, von Zglinicki T (2019) Senolytics and senostatics as adjuvant tumour therapy. EBioMedicine 41:683–692. https://doi.org/10.1016/j.ebiom.2019.01.056
Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Hara SPO et al (2015) The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14:644–658. https://doi.org/10.1111/acel.12344
Saleh T, Tyutyunyk-Massey L, Gewirtz DADA (2019) Tumor cell escape from therapy-induced senescence as a model of disease recurrence after dormancy. Cancer Res 79:1044–1046. https://doi.org/10.1158/0008-5472.CAN-18-3437
Saleh T, Gewirtz DA (2022) Considering therapy-induced senescence as a mechanism of tumour dormancy contributing to disease recurrence. Br J Cancer 126:1363–1365. https://doi.org/10.1038/S41416-022-01787-6
DeLuca VJ, Saleh T (2023) Insights into the role of senescence in tumor dormancy: mechanisms and applications. Cancer Metastasis Rev. https://doi.org/10.1007/S10555-023-10082-6
Saleh T, Alhesa A, Al-Balas M, Abuelaish O, Mansour A, Awad H, El-Sadoni M, Carpenter VJ, Azab B (2021) Expression of therapy-induced senescence markers in breast cancer samples upon incomplete response to neoadjuvant chemotherapy. Biosci Rep 41:BSR20210079. 10. 1042/ bsr 2021 0079
Saleh T, El-sadoni M, Alhesa A, Awad H, Jaradat M, Al-hazaimeh M, Dawoud R, Mryyian A, Azab B (2021) Expression of senescence and apoptosis biomarkers in synchronous bilateral breast cancer: a case report. Curr Oncol 28:3836. https://doi.org/10.3390/CURRONCOL28050327
Saleh T, Alhesa A, El-Sadoni M, Shahin NA, Alsharaiah E, Shboul SA, Awad H, Bloukh S, Al-Balas M, Alsalem M et al (2022) The Expression of the senescence-associated biomarker lamin B1 in human breast cancer. Diagnostics (Basel, Switzerland) 12:609. https://doi.org/10.3390/DIAGNOSTICS12030609
Freund A, Laberge R-MRM, Demaria M, Campisi J (2012) Lamin B1 loss is a senescence-associated biomarker. Mol Biol Cell 23:2066–2075. https://doi.org/10.1091/mbc.E11-10-0884
Shimi T, Butin-Israeli V, Adam SA, Hamanaka RB, Goldman AE, Lucas CA, Shumaker DK, Kosak ST, Chandel NS, Goldman RD (2011) The role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev. https://doi.org/10.1101/gad.179515.111
Chandra T, Ewels PA, Schoenfelder S, Furlan-Magaril M, Wingett SW, Kirschner K, Thuret JY, Andrews S, Fraser P, Reik W (2015) Global reorganization of the nuclear landscape in senescent cells. Cell Rep 10:471–483. https://doi.org/10.1016/j.celrep.2014.12.055
González-Gualda E, Baker AG, Fruk L, Muñoz-Espín D (2020) A guide to assessing cellular senescence in vitro and in vivo. FEBS J. https://doi.org/10.1111/febs.15570
Radisky DC, Santisteban M, Berman HK, Gauthier ML, Frost MH, Reynolds CA, Vierkant RA, Pankratz VS, Visscher DW, Tlsty TD et al (2011) P16 INK4a expression and breast cancer risk in women with atypical hyperplasia. Cancer Prev Res. https://doi.org/10.1158/1940-6207.CAPR-11-0282
Rayess H, Wang MB, Srivatsan ES (2012) Cellular senescence and tumor suppressor gene P16. Int J Cancer 130:1715–1725. https://doi.org/10.1002/ijc.27316
Shan M, Zhang X, Liu X, Qin Y, Liu T, Liu Y, Wang J, Zhong Z, Zhang Y, Geng J et al (2013) P16 and P53 play distinct roles in different subtypes of breast cancer. PLoS ONE 8:e76408
Shin E, Jung W-H, Koo J-S (2015) Expression of P16 and PRB in invasive breast cancer. Int J Clin Exp Pathol 8:8209
Lee WJ, Škalamera D, Dahmer-Heath M, Shakhbazov K, Ranall MV, Fox C, Lambie D, Stevenson AJ, Yaswen P, Gonda TJ et al (2017) Genome-wide overexpression screen identifies genes able to bypass P16-mediated senescence in melanoma. SLAS Discovery 22:298–308. https://doi.org/10.1177/1087057116679592
Inwald EC, Klinkhammer-Schalke M, Hofstädter F, Zeman F, Koller M, Gerstenhauer M, Ortmann O (2013) Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat 139:539. https://doi.org/10.1007/S10549-013-2560-8
Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP (2017) The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 67:93–99. https://doi.org/10.3322/CAAC.21388
Al Shboul S, Curran OE, Alfaro JA, Lickiss F, Nita E, Kowalski J, Naji F, Nenutil R, Ball KL, Krejcir R et al (2021) Kinomics platform using GBM tissue identifies BTK as being associated with higher patient survival. Life Sci Alliance 4:e202101054. https://doi.org/10.26508/LSA.202101054
Edgar R, Domrachev M, Lash AE (2002) Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucl Acids Res 30:207–210. https://doi.org/10.1093/NAR/30.1.207
Vera-Ramirez L, Sanchez-Rovira P, Ramirez-Tortosa CL, Quiles JL, Ramirez-Tortosa MC, Lorente JA (2013) Transcriptional shift identifies a set of genes driving breast cancer chemoresistance. PLoS ONE 8:e53983. https://doi.org/10.1371/JOURNAL.PONE.0053983
Stickeler E, Pils D, Klar M, Orlowsk-Volk M, Zur Hausen A, Jäger M, Watermann D, Gitsch G, Zeillinger R, Tempfer CB (2011) Basal-like molecular subtype and HER4 up-regulation and response to neoadjuvant chemotherapy in breast cancer. Oncol Rep 26:1037–1045. https://doi.org/10.3892/OR.2011.1392
Radspieler MM, Schindeldecker M, Stenzel P, Försch S, Tagscherer KE, Herpel E, Hohenfellner M, Hatiboglu G, Roth W, Macher-Goeppinger S (2019) Lamin-B1 is a senescence-associated biomarker in clear-cell renal cell carcinoma. Oncol Lett. https://doi.org/10.3892/ol.2019.10593
Galvis D, Walsh D, Harries LW, Latorre E, Rankin J (2019) A dynamical systems model for the measurement of cellular senescence. J R Soc Interface 16:20190311. https://doi.org/10.1098/rsif.2019.0311
Zindy F, Quelle DE, Roussel MF, Sherr CJ (1997) Expression of the P16(INK4a) tumor suppressor versus other INK4 family members during mouse development and aging. Oncogene 15:203–211
Takahashi A, Ohtani N, Hara E (2007) Irreversibility of cellular senescence: dual roles of P16INK4a/Rb-pathway in cell cycle control. Cell Div 2:1–5. https://doi.org/10.1186/1747-1028-2-10
Liu JY, Souroullas GP, Diekman BO, Krishnamurthy J, Hall BM, Sorrentino JA, Parker JS, Sessions GA, Gudkov AV, Sharpless NE (2019) Cells exhibiting strong P16 INK4a promoter activation in vivo display features of senescence. Proc Natl Acad Sci USA 116:2603–2611. https://doi.org/10.1073/pnas.1818313116
Biran A, Zada L, Abou Karam P, Vadai E, Roitman L, Ovadya Y, Porat Z, Krizhanovsky V (2017) Quantitative identification of senescent cells in aging and disease. Aging Cell 16:661–671. https://doi.org/10.1111/acel.12592
Wang AS, Ong PF, Chojnowski A, Clavel C, Dreesen O (2017) Loss of lamin B1 is a biomarker to quantify cellular senescence in photoaged skin. Sci Rep 7:15678. https://doi.org/10.1038/S41598-017-15901-9
Wang B, Demaria M (2021) The quest to define and target cellular senescence in cancer. Cancer Res 81:6087–6089. https://doi.org/10.1158/0008-5472.CAN-21-2032
Troiani M, Colucci M, D’Ambrosio M, Guccini I, Pasquini E, Varesi A, Valdata A, Mosole S, Revandkar A, Attanasio G et al (2022) Single-cell transcriptomics identifies Mcl-1 as a target for senolytic therapy in cancer. Nat Commun 13:2177. https://doi.org/10.1038/S41467-022-29824-1
Milanovic M, Fan DNY, Belenki D, Däbritz JHM, Zhao Z, Yu Y, Dörr JR, Dimitrova L, Lenze D, Monteiro Barbosa IA et al (2018) Senescence-associated reprogramming promotes cancer stemness. Nature 553:96–100. https://doi.org/10.1038/nature25167
Tonnessen-Murray CA, Frey WD, Rao SG, Shahbandi A, Ungerleider NA, Olayiwola JO, Murray LB, Vinson BT, Chrisey DB, Lord CJ et al (2019) Chemotherapy-induced senescent cancer cells engulf other cells to enhance their survival. J Cell Biol 218:3827–3844. https://doi.org/10.1083/jcb.201904051
Sasaki M, Kumazaki T, Takano H, Nishiyama M, Mitsui Y (2001) Senescent cells are resistant to death despite low Bcl-2 level. Mech Ageing Dev 122:1695–1706. https://doi.org/10.1016/S0047-6374(01)00281-0
Hampel B, Wagner M, Teis D, Zwerschke W, Huber LA, Jansen-Dürr P (2005) Apoptosis resistance of senescent human fibroblasts is correlated with the absence of nuclear IGFBP-3. Aging Cell 4:325–330. https://doi.org/10.1111/j.1474-9726.2005.00180.x
Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O (2009) Protocols to detect senescence-associated beta-galactosidase (SA-Betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc 4:1798–1806. https://doi.org/10.1038/NPROT.2009.191
Noren Hooten N, Evans MK (2017) Techniques to induce and quantify cellular senescence. J Vis Exp. https://doi.org/10.3791/55533
Kohli J, Wang B, Brandenburg SM, Basisty N, Evangelou K, Varela-Eirin M, Campisi J, Schilling B, Gorgoulis V, Demaria M (2021) Algorithmic assessment of cellular senescence in experimental and clinical specimens. Nat Protoc 16:2471–2498. https://doi.org/10.1038/S41596-021-00505-5
Park SS, Choi YW, Kim JH, Kim HS, Park TJ (2021) Senescent tumor cells: an overlooked adversary in the battle against cancer. Exp Mol Med 53:1834–1841. https://doi.org/10.1038/S12276-021-00717-5
Georgakopoulou EA, Tsimaratou K, Evangelou K, Fernandez-Marcos PJ, Zoumpourlis V, Trougakos IP, Kletsas D, Bartek J, Serrano M, Gorgoulis VG (2013) Specific lipofuscin staining as a novel biomarker to detect replicative and stress-induced senescence a method applicable in cryo-preserved and archival tissues. Aging (Albany NY) 5:37–50. https://doi.org/10.18632/aging.100527
Yang N, Hu M (2005) The limitations and validities of senescence associated-b-galactosidase activity as an aging marker for human foreskin fibroblast Hs68 cells. Exp Gerontol 40:813–819. https://doi.org/10.1016/j.exger.2005.07.011
Jannone G, Rozzi M, Najimi M, Decottignies A, Sokal EM (2020) An optimized protocol for histochemical detection of senescence-associated beta-galactosidase activity in cryopreserved liver tissue. J Histochem Cytochem 68:269–278. https://doi.org/10.1369/0022155420913534
Wagner J, Damaschke N, Yang B, Truong M, Guenther C (2015) Overexpression of the novel senescence marker β-galactosidase (GLB1) in prostate cancer predicts reduced PSA recurrence. PLOS Biol 10:1–15. https://doi.org/10.1371/journal.pone.0124366
Tran SL, Haferkamp S, Scurr LL, Gowrishankar K, Becker TM, Desilva C, Thompson JF, Scolyer RA, Kefford RF, Rizos H (2012) Absence of distinguishing senescence traits in human melanocytic Nevi. J Investig Dermatol 132:2226–2234. https://doi.org/10.1038/jid.2012.126
Shah PP, Donahue G, Otte GL, Capell BC, Nelson DM, Cao K, Aggarwala V, Cruickshanks HA, Rai TS, McBryan T et al (2013) Lamin B1 depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape. Genes Dev 27:1787–1799. https://doi.org/10.1101/gad.223834.113
Sliwinska MA, Mosieniak G, Wolanin K, Babik A, Piwocka K, Magalska A, Szczepanowska J, Fronk J, Sikora E (2009) Induction of senescence with doxorubicin leads to increased genomic instability of HCT116 cells. Mech Ageing Dev 130:24–32. https://doi.org/10.1016/j.mad.2008.04.011
Sobecki M, Mrouj K, Colinge J, Gerbe F, Jay P, Krasinska L, Dulic V, Fisher D (2017) Cell-cycle regulation accounts for variability in Ki-67 expression levels. Cancer Res 77:2722–2734. https://doi.org/10.1158/0008-5472.CAN-16-0707
Dowsett M, Dunbier AK (2008) Emerging biomarkers and new understanding of traditional markers in personalized therapy for breast cancer. Clinical 14:8019–8026. https://doi.org/10.1158/1078-0432.CCR-08-0974
Haugstetter AM, Loddenkemper C, Lenze D, Gröne J, Standfu C, Petersen I, Dörken B, Schmitt CA, Standfuß C, Petersen I et al (2010) Cellular senescence predicts treatment outcome in metastasised colorectal cancer. Br J Cancer 103:505–509. https://doi.org/10.1038/sj.bjc.6605784
Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW (1997) Oncogenic Ras provokes premature cell senescence associated with accumulation of P53 and P16INK4a. Cell 88:593–602. https://doi.org/10.1016/S0092-8674(00)81902-9
Lin AW, Barradas M, Stone JC, Van Aelst L, SerranoLowe MSW (1998) Premature senescence involving P53 and P16 is activated in response to constitutive MEK/MAPK mitogenic signaling. GENES Dev 12:3008–3019
Carpenter VJ, Patel BB, Autorino R, Smith SC, Gewirtz DA, Saleh T (2020) Senescence and castration resistance in prostate cancer: a review of experimental evidence and clinical implications. Biochim Biophys Acta Rev Cancer 1874:188424. https://doi.org/10.1016/J.BBCAN.2020.188424
Okamoto A, Demetrick DJ, Spillare EA, Hagiwara K, Hussain SP, Bennett WP, Forrester K, Gerwin B, Serrano M, Beach DH (1994) Mutations and altered expression of P16INK4 in human cancer. Proc Natl Acad Sci 91:11045–11049. https://doi.org/10.1073/pnas.91.23.11045
Giatromanolaki A, Kouroupi M, Balaska K, Koukourakis MI (2020) Immunohistochemical detection of senescence markers in human sarcomas. Pathol Res Pract 216:152800. https://doi.org/10.1016/J.PRP.2019.152800
Evangelou K, Gorgoulis VG, Sudan Black B (2017) The specific histochemical stain for lipofuscin: a novel method to detect senescent cells. Methods Mol Biol 1534:111–119. https://doi.org/10.1007/978-1-4939-6670-7_10
Sirinian C, Peroukidis S, Kriegsmann K, Chaniotis D, Koutras A, Kriegsmann M, Papanastasiou AD (2022) Cellular senescence in normal mammary gland and breast cancer. Implications for cancer therapy. Genes (Basel) 13:994. https://doi.org/10.3390/GENES13060994
Hoenicke L, Zender L (2012) Immune surveillance of senescent cells—biological significance in cancer- and non-cancer pathologies. Carcinogenesis 33:1123–1126. https://doi.org/10.1093/carcin/bgs124
Park MH, Choi JE, Kim JR, Bae YK (2021) Immunohistochemical expressions of senescence-associated secretory phenotype and its association with immune microenvironments and clinicopathological factors in invasive breast cancer. Pathol Oncol Res 27:1609795. https://doi.org/10.3389/PORE.2021.1609795
Milde-Langosch K, Bamberger AM, Rieck G, Kelp B, Löning T (2001) Overexpression of the P16 cell cycle inhibitor in breast cancer is associated with a more malignant phenotype. Breast Cancer Res Treat 67:61–70. https://doi.org/10.1023/A:1010623308275
Nielsen TO, Leung SCY, Rimm DL, Dodson A, Acs B, Badve S, Denkert C, Ellis MJ, Fineberg S, Flowers M et al (2020) Assessment of Ki67 in breast cancer: updated recommendations from the international Ki67 in Breast Cancer Working Group. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djaa201
Penault-Llorca F, André F, Sagan C, Lacroix-Triki M, Denoux Y, Verriele V, Jacquemier J, Baranzelli MC, Bibeau F, Antoine M et al (2009) Ki67 expression and docetaxel efficacy in patients with estrogen receptor-positive breast cancer. J Clin Oncol 27:2809–2815. https://doi.org/10.1200/JCO.2008.18.2808
Viale G, Regan MM, Mastropasqua MG, Maffini F, Maiorano E, Colleoni M, Price KN, Golouh R, Perin T, Brown RW et al (2008) Predictive value of tumor Ki-67 expression in two randomized trials of adjuvant chemoendocrine therapy for node-negative breast cancer. J Natl Cancer Inst 100:207–212. https://doi.org/10.1093/JNCI/DJM289
Wazir U, Ahmed MH, Bridger JM, Harvey A, Jiang WG, Sharma AK, Mokbel K (2013) The Clinicopathological significance of lamin A/C, lamin B1 and lamin B receptor MRNA expression in human breast cancer. Cell Mol Biol Lett 18:595–611. https://doi.org/10.2478/s11658-013-0109-9
Pereira BI, Devine OP, Vukmanovic-Stejic M, Chambers ES, Subramanian P, Patel N, Virasami A, Sebire NJ, Kinsler V, Valdovinos A et al (2019) Senescent cells evade immune clearance via HLA-E-mediated NK and CD8+ T cell inhibition. Nat Commun 10:2387. https://doi.org/10.1038/s41467-019-10335-5
Carpenter VJ, Saleh T, Gewirtz DA (2021) Senolytics for cancer therapy: is all that glitters really gold? Cancers (Basel) 13:723. https://doi.org/10.3390/cancers13040723
Chen W, Wang X, Wei G, Huang Y, Shi Y, Li D, Qiu S, Zhou B, Cao J, Chen M et al (2020) Single-cell transcriptome analysis reveals six subpopulations reflecting distinct cellular fates in senescent mouse embryonic fibroblasts. Front Genet 11:867. https://doi.org/10.3389/fgene.2020.00867
Almanzar N, Antony J, Baghel AS, Bakerman I, Bansal I, Barres BA, Beachy PA, Berdnik D, Bilen B, Brownfield D et al (2020) A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature 583:590–595. https://doi.org/10.1038/s41586-020-2496-1
Althubiti M, Lezina L, Carrera S, Jukes-Jones R, Giblett SM, Antonov A, Barlev N, Saldanha GS, Pritchard CA, Cain K et al (2014) Characterization of novel markers of senescence and their prognostic potential in cancer. Cell Death Dis. https://doi.org/10.1038/cddis.2014.489
Abramovitz M, Barwick BG, Willis S, Young B, Catzavelos C, Li Z, Kodani M, Tang W, Bouzyk M, Moreno CS et al (2011) Molecular characterisation of formalin-fixed paraffin-embedded (FFPE) breast tumour specimens using a custom 512-gene breast cancer bead array-based platform. Br J Cancer 105:1574–1581. https://doi.org/10.1038/BJC.2011.355
Fridman AL, Tainsky MA (2008) Critical pathways in cellular senescence and immortalization revealed by gene expression profiling. Oncogene 27:5975–5987. https://doi.org/10.1038/onc.2008.213
Acknowledgements
The authors would like to thank the Cell Therapy Center (University of Jordan) for granting access to the use of the microscopic imaging facility. The authors would also like to thank Dr. AbdelKader Battah and Dr. Heyam Awad (The School of Medicine, The University of Jordan) for their kind assistance and directions throughout this project. This work was funded by the Master Student Funding Program by The Deanship of Scientific Research, The University of Jordan (Grant no. 26/2020-2021). Work in Dr. Tareq Saleh’s laboratory is supported by Deanship of Scientific Research, The Hashemite University (Grants no. 465/83/2019 and 418/84/2019). All authors are highly thankful to the Researchers Supporting Project number (RSPD-2023R786), King Saud University, Riyadh, Saudi Arabia.
Funding
This work was funded by the Master Student Funding Program by The Deanship of Scientific Research, The University of Jordan (Grant no. 26/2020–2021). Work in Dr. Tareq Saleh’s laboratory is supported by Deanship of Scientific Research, The Hashemite University (Grants no. 465/83/2019 and 418/84/2019). All authors are highly thankful to the Researchers Supporting Project number (RSPD-2023R786), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
ME-S and AA collected patients’ samples and performed all laboratory experiments including tissue processing and immunostaining. SAS performed data analysis, designed tables and figures, and contributed to the writing. NAS and EA performed pathological assessment including diagnosis and evaluation of protein marker expression. MAI and NAA performed microscopic procedures. Manuscript was revised and edited by BA and MRA. TS was responsible for the experimental design, supervised the work, and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that all research activities were conducted in the absence of any commercial or financial conflicts of interest.
Ethics approval and consent to participate
All experimental procedures were approved by the Institutional Review Board (IRB) committees at both JRMS (6/2021), the Hashemite University (no. 3/5/2018/2019), and The University of Jordan (no. 237/2021) in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. Obtaining informed consents for this work was waived by both IRB protocols since all the samples used for this study were surplus (archived) tumor tissue samples, and that patients undergoing surgery or biopsy collection provide informed consent to donate any excess tissue (i.e., beyond that needed for clinical purposes).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
El-Sadoni, M., Shboul, S.A., Alhesa, A. et al. A three-marker signature identifies senescence in human breast cancer exposed to neoadjuvant chemotherapy. Cancer Chemother Pharmacol 91, 345–360 (2023). https://doi.org/10.1007/s00280-023-04523-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-023-04523-w