Abstract
Purpose
The objective of this analysis was to develop a population pharmacokinetic (PPK) model to characterize camidanlumab tesirine (Cami) pharmacokinetics based on the phase 1 study in relapsed/refractory lymphoma (NCT02432235).
Methods
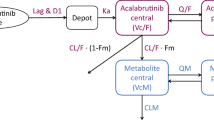
An initial PPK model was developed based on a two-compartment model with parallel linear and nonlinear elimination pathways. Pharmacokinetic parameters were evaluated for correlation with potential demographic covariates; significant covariates were retained in the final model.
Results
In the final PPK model, baseline weight effects were included on clearance (CL), intercompartmental clearance (Q), and the volumes of distribution in the central (V1) and peripheral (V2) compartments. The baseline soluble CD25 (sCD25) effect was included on CL and maximum velocity of saturable clearance (Vmax); sex effect was included on CL and V1; and ethnicity effect was included on deconjugation clearance (CLdec). For a typical patient, CL and CLdec were 0.516 and 0.21 L/day, respectively (tAb elimination half-life: 18.72 days); V1 and V2 were 4.41 and 2.67 L, respectively; Vmax was 0.49 mg/day; the Michaelis–Menten constant (Km) was 0.409 µg/mL; and the first-order rate for decrease of Vmax (KDES) was 0.0197/day. Cami exposure was higher for patients with low baseline sCD25, higher body weight, and females.
Conclusions
The final model described the observed data well, estimates of PK parameters were obtained, and covariates with significant effects on Cami exposure were identified. Altogether, this final PPK model provides a robust basis for analysis of Cami exposure–response relationships and further supports identification of the optimal Cami dosing schedule for patients with relapsed/refractory lymphoma.



Similar content being viewed by others
Data Availability
Summary trial information for study NCT02432235 is available online. Requests regarding data collected for the study should be sent to clinical.trials@adctherapeutics.com.
References
Jiang M, Bennani NN, Feldman AL (2017) Lymphoma classification update: T-cell lymphomas, Hodgkin lymphomas, and histiocytic/dendritic cell neoplasms. Expert Rev Hematol 10:239–249. https://doi.org/10.1080/17474086.2017.1281122
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R et al (2016) The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 127:2375–2390. https://doi.org/10.1182/blood-2016-01-643569
Al-Hamadani M, Habermann TM, Cerhan JR, Macon WR, Maurer MJ, Go RS (2015) Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: a longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am J Hematol 90:790–795. https://doi.org/10.1002/ajh.24086
Kaseb H, Babiker HM (2021) Hodgkin lymphoma. In: Statpearls. StatPearls Publishing. StatPearls Publishing LLC., Treasure Island
LaCasce AS (2019) Treating Hodgkin lymphoma in the new millennium: Relapsed and refractory disease. Hematol Oncol 37(Suppl 1):87–91. https://doi.org/10.1002/hon.2589
Sheikh S, Kuruvilla J (2021) Addressing an unmet need in relapsed or refractory Hodgkin lymphoma. JCO Oncol Pract 17:74–76. https://doi.org/10.1200/op.20.01029
Bentolila G, Pavlovsky A (2020) Relapse or refractory Hodgkin lymphoma: determining risk of relapse or progression after autologous stem-cell transplantation. Leukemia Lymphoma 61:1548–1554. https://doi.org/10.1080/10428194.2020.1732959
Flynn MJ, Hartley JA (2017) The emerging role of anti-CD25 directed therapies as both immune modulators and targeted agents in cancer. Br J Haematol 179:20–35. https://doi.org/10.1111/bjh.14770
Zain JM (2019) Aggressive T-cell lymphomas: 2019 updates on diagnosis, risk stratification, and management. Am J Hematol 94:929–946. https://doi.org/10.1002/ajh.25513
Hartley JA (2021) Antibody-drug conjugates (ADCs) delivering pyrrolobenzodiazepine (PBD) dimers for cancer therapy. Expert Opin Biol Ther 21:931–943. https://doi.org/10.1080/14712598.2020.1776255
Flynn MJ, Zammarchi F, Tyrer PC, Akarca AU, Janghra N, Britten CE et al (2016) ADCT-301, a pyrrolobenzodiazepine (PBD) dimer-containing antibody-drug conjugate (ADC) targeting CD25-expressing hematological malignancies. Mol Cancer Ther 15:2709–2721. https://doi.org/10.1158/1535-7163.MCT-16-0233
Hartley JA (2011) The development of pyrrolobenzodiazepines as antitumour agents. Expert Opin Investig Drugs 20:733–744. https://doi.org/10.1517/13543784.2011.573477
Mahmood I (2021) Effect of intrinsic and extrinsic factors on the pharmacokinetics of antibody-drug conjugates (ADCs). Antibodies (Basel). https://doi.org/10.3390/antib10040040
Hamadani M, Collins GP, Caimi PF, Samaniego F, Spira A, Davies A et al (2021) Camidanlumab tesirine in patients with relapsed or refractory lymphoma: A phase 1, open-label, multicentre, dose-escalation, dose-expansion study. Lancet Haematol 8:e433–e445. https://doi.org/10.1016/S2352-3026(21)00103-4
Goldberg AD, Atallah E, Rizzieri D, Walter RB, Chung KY, Spira A et al (2020) Camidanlumab tesirine, an antibody-drug conjugate, in relapsed/refractory CD25-positive acute myeloid leukemia or acute lymphoblastic leukemia: a phase I study. Leukemia Res 95:106385. https://doi.org/10.1016/j.leukres.2020.106385
Malik PRV, Temrikar ZH, Chelle P, Edginton AN, Meibohm B (2021) Pediatric dose selection for therapeutic proteins. J Clin Pharmacol 61(Suppl 1):S193-s206. https://doi.org/10.1002/jcph.1829
Toukam M, Boni JP, Hamadani M, Caimi PF, Cruz HG, Wuerthner J. Exposure-response analysis of Camidanlumab tesirine in patients with relapsed or refractory classical Hodgkin lymphoma and non-Hodgkin lymphoma. Cancer Chemother Pharmacol. https://doi.org/10.1007/s00280-022-04487-3
(2020) Drug development and drug interactions: table of substrates, inhibitors and inducers
(2020) Clinical drug interaction studies—cytochrome p450 enzyme- and transporter-mediated drug interactions guidance for industry
Population pharmacokinetic analysis report of ADCT-301 in patients with relapsed or refractory Hodgkin lymphoma and non-Hodgkin lymphoma
(1999) Guidance for Industry: population pharmacokinetics
(2007) Guideline on reporting the results of population pharmacokinetic analyses. London, UK
Gibiansky L, Gibiansky E (2014) Target-mediated drug disposition model and its approximations for antibody-drug conjugates. J Pharmacokinet Pharmacodyn 41:35–47. https://doi.org/10.1007/s10928-013-9344-y
Krause A, Lott D, Dingemanse J (2021) Estimation of attainment of steady-state conditions for compounds with a long half-life. J Clin Pharmacol 61:82–89. https://doi.org/10.1002/jcph.1701
Acknowledgements
Medical writing support was provided by Adrianne Spencer, Ph.D. (CiTRUS Health Group), and was funded by ADC Therapeutics (Murray Hill, NJ) in accordance with Good Publication Practice (GPP3) guidelines.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M Toukam is currently employed and a current equity holder at ADC Therapeutics America, Inc., a publicly traded company. J Wuerthner is currently employed and a current equity holder at ADC Therapeutics SA, a publicly traded company. K Havenith is currently employed and a current equity holder at ADC Therapeutics (UK) Ltd, a publicly traded company. M Hamadani reports consultancy with Janssen R&D, Incyte Corporation, ADC Therapeutics, Celgene Corporation, Pharmacyclics, Omeros, AbGenomics, Verastem, TeneoBio, Seagen, Genmab, Novartis, and Kite; membership on an entity’s board of directors or advisory committees for ADC Therapeutics; research funding from Takeda Pharmaceutical Company, Spectrum Pharmaceuticals, and Astellas Pharma; and speakers bureau memberships for Sanofi Genzyme, AstraZeneca, BeiGene, and Kite. PF Caimi reports advisory board memberships for Amgen, Bayer, Kite Pharma, ADC Therapeutics, Novartis, and Genentech; research funding for ADC Therapeutics and Genentech; and speakers bureau membership for Celgene. T Kopotsha is currently employed and a current equity holder at ADC Therapeutics (UK) Ltd, a publicly traded company. HG Cruz is currently employed and a current equity holder at ADC Therapeutics SA, a publicly traded company. JP Boni is currently employed and a current equity holder at ADC Therapeutics America, Inc., a publicly traded company.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Toukam, M., Wuerthner, J., Havenith, K. et al. Population pharmacokinetics analysis of camidanlumab tesirine in patients with relapsed or refractory Hodgkin lymphoma and non-Hodgkin lymphoma. Cancer Chemother Pharmacol 91, 13–24 (2023). https://doi.org/10.1007/s00280-022-04486-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-022-04486-4