Abstract
Background
Notch signaling plays an integral role in development and tissue homeostasis. Inhibition of Notch signaling has been identified as a reasonable target for oncotherapy. Crenigacestat (LY3039478) is a potent Notch inhibitor that decreases Notch signaling and its downstream biologic effects.
Methods
I6F-MC-JJCD was a multicenter, nonrandomized, open-label, phase 1b study with 5 separate, parallel dose escalations in patients with advanced or metastatic cancer from a variety of solid tumors followed by a dose-confirmation phase in pre-specified tumor types. This manuscript reports on 2 of 5 groups. The primary objective was to determine the recommended phase 2 dose of crenigacestat combined with other anticancer agents (gemcitabine/cisplatin or gemcitabine/carboplatin). Secondary objectives included evaluation of safety, tolerability, preliminary efficacy, and pharmacokinetics.
Results
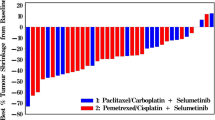
Patients (N = 31) received treatment between November 2016 and July 2019. Dose-limiting toxicities occurred in 6 patients. The recommended phase 2 dose for crenigacestat was 50 mg TIW in Part 1 (combined with gemcitabine/cisplatin) and not established in Part 2 (combined with gemcitabine/carboplatin) due to poor tolerability. Patients had at least one treatment-emergent adverse event (TEAE), and most had Grade ≥ 3 TEAEs. Over 50% of the patients experienced gastrointestinal disorders (Grade ≥ 3). No patient had complete response; 5 patients had a partial response. Disease control rates were 62.5% (Part 1) and 60.0% (Part 2).
Conclusion
This study demonstrated that the Notch inhibitor, crenigacestat, combined with different anticancer agents (gemcitabine, cisplatin, and carboplatin) was poorly tolerated and resulted in disappointing clinical activity in patients with advanced or metastatic solid tumors.
Clinicaltrials.gov Identification Number: NCT02784795.

Similar content being viewed by others
Availability of data
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
References
Artavanis-Tsakonas S, Rand MD, Lake RJ (1999) Notch signaling: cell fate control and signal integration in development. Science 284:770–776. https://doi.org/10.1126/science.284.5415.770
Allenspach EJ, Maillard I, Aster JC, Pear WS (2002) Notch signaling in cancer. Cancer Biol Ther 1:466–476. https://doi.org/10.4161/cbt.1.5.159
Radtke F, Raj K (2003) The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nat Rev Cancer 3:756–767. https://doi.org/10.1038/nrc1186
Koch U, Radtke F (2007) Notch and cancer: a double-edged sword. Cell Mol Life Sci 64:2746–2762. https://doi.org/10.1007/s00018-007-7164-1
Cancer Genome Atlas Research N (2011) Integrated genomic analyses of ovarian carcinoma. Nature 474:609–615. https://doi.org/10.1038/nature10166
Puente XS, Pinyol M, Quesada V et al (2011) Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature 475:101–105. https://doi.org/10.1038/nature10113
Bender MH, Gao H, Capen AR et al (2013) Abstract 1131: novel inhibitor of Notch signaling for the treatment of cancer. Cancer Res 73:1131
Garber K (2007) Notch emerges as new cancer drug target. J Natl Cancer Inst 99:1284–1285. https://doi.org/10.1093/jnci/djm148
van Es JH, van Gijn ME, Riccio O et al (2005) Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435:959–963. https://doi.org/10.1038/nature03659
Zecchini V, Domaschenz R, Winton D et al (2005) Notch signaling regulates the differentiation of postmitotic intestinal epithelial cells. Genes Dev 19:1686–1691. https://doi.org/10.1101/gad.341705
Real PJ, Tosello V, Palomero T et al (2009) Gamma-secretase inhibitors reverse glucocorticoid resistance in T-cell acute lymphoblastic leukemia. Nat Med 15(1):50–58. https://doi.org/10.1038/nm.1900
Massard C, Azaro A, Soria JC et al (2018) First-in-human study of LY3039478, an oral Notch signaling inhibitor in advanced or metastatic cancer. Ann Oncol 29(9):1911–1917. https://doi.org/10.1093/annonc/mdy244
Evan C, Lassen U, Merchan J et al (2020) Safety and clinical activity of the Notch inhibitor, crenigacestat (LY3039478), in an open-label phase I trial expansion cohort of advanced or metastatic adenoid cystic carcinoma. Invest New Drugs 38(2):402–409. https://doi.org/10.1007/s10637-019-00739-x
Gemcitabine [package insert], Eli Lilly and Company, Indianapolis, Indiana; 1996.
Wang Z, Li Y, Kong D et al (2009) Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res 69:2400–2407. https://doi.org/10.1158/0008-5472
Wang Z, Li Y, Ahmad A et al (2010) Targeting Notch signaling pathway to overcome drug-resistance for cancer therapy. Biochim Biophys Acta 1806(2):258–267. https://doi.org/10.1016/j.bbcan.2010.06.001
Meng RD, Shelton CC, Li YM et al (2009) gamma- Secretase inhibitors abrogate oxaliplatin-induced activation of the Notch-1 signaling pathway in colon cancer cells resulting in enhanced chemosensitivity. Cancer Res 69(2):573–582. https://doi.org/10.1158/0008-5472
Yao J, Qian C (2010) Inhibition of Notch3 enhances sensitivity to gemcitabine in pancreatic cancer through an inactivation of PI3K/Akt-dependent pathway. Med Oncol 27(3):1017–1022. https://doi.org/10.1007/s12032-009-9326-5
Zang S, Chen F, Dai J et al (2010) RNAi-mediated knockdown of Notch-1 leads to cell growth inhibition and enhanced chemosensitivity in human breast cancer. Oncol Rep 23:893–899. https://doi.org/10.3892/or_00000712
Cisplatin [package insert], WG Critical Care, LLC, Paramus, New Jersey; 2015.
Carboplatin [package insert], Teva Parenteral Medicines, Inc., Irvine, California; 2011.
Dasari S, Tchounwou PB (2014) Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 740:364–378. https://doi.org/10.1016/j.ejphar.2014.07.025
Mokhtari RB, Homayouny TS, Baluch N et al (2017) Combination therapy in combating cancer. Oncotarget 8(23):38022–38043. https://doi.org/10.18632/oncotarget.16723
Gu F, Ma Y, Zhang Z et al (2010) Expression of Stat3 and Notch1 is associated with cisplatin resistance in head and neck squamous cell carcinoma. Oncol Rep 23:671–676. https://doi.org/10.3892/or_00000683
Aleksic T, Feller SM (2008) Gamma-secretase inhibition combined with platinum compounds enhances cell death in a large subset of colorectal cancer cells. Cell Commun Signal 6:8. https://doi.org/10.1186/1478-811X-6-8
O’Shaughnessy J, Osborne C, Pippen JE et al (2011) Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med 364(3):205–214. https://doi.org/10.1056/NEJMoa1011418
Eckel F, Brunner T, Jelic S, Group EGW (2011) Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 22(Suppl 6):vi40–vi44. https://doi.org/10.1093/annonc/mdr375
Network NNCC. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Hepatobiliary Cancers. 2015. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx#hepatobiliary. Accessed 24 January 2020.
Zender S, Nickeleit I, Wuestefeld T et al (2013) A critical role for notch signaling in the formation of cholangiocellular carcinomas. Cancer Cell 23:784–795. https://doi.org/10.1016/j.ccr.2013.04.019
Azaro A, Massard C, Tap WD et al (2021) A phase 1b study of the Notch inhibitor crenigacestat (LY3039478) in combination with other anticancer target agents (taladegib, LY3023414, or abemaciclib) in patients with advanced or metastatic solid tumors. Invest New Drugs 39(4):1089–1098. https://doi.org/10.1007/s10637-021-01094-6
Choi H, Charnsangavej C, Faria SC et al (2007) Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol 25(13):1753–1759. https://doi.org/10.1200/JCO.2006.07.3049
Serdjebi C, Gattacceca F, Seitz JF et al (2017) Population pharmacokinetics of gemcitabine and dFdU in pancreatic cancer patients using an optimal design. Sparse Sampl Appr Ther Drug Monit 39(3):290–296. https://doi.org/10.1097/FTD.0000000000000399
Urien S, Brain E, Bugat R et al (2005) Pharmacokinetics of platinum after oral or intravenous cisplatin: a phase 1 study in 32 adult patients. Cancer Chemother Pharmacol 55(1):55–60. https://doi.org/10.1007/s00280-004-0852-8
Joerger M, Huitema AD, Richel DJ et al (2007) Population pharmacokinetics and pharmacodynamics of paclitaxel and carboplatin in ovarian cancer patients: a study by the european organization for research and treatment of cancer-pharmacology and molecular Mechanisms Group and New Drug Development Group. Clin Cancer Res 13(21):6410–6418. https://doi.org/10.1158/1078-0432.CCR-07-0064
Acknowledgements
Medical writing support was provided by Debra Hidayetoglu, on behalf of Covance, Inc.
Funding
This trial was funded by Eli Lilly and Company.
Author information
Authors and Affiliations
Contributions
All authors meet the International Committee of Medical Journal Editors criteria for authorship for this manuscript, take responsibility for the integrity of the work, and have given final approval of the version of the manuscript to be published.
Corresponding author
Ethics declarations
Conflict of interest
A Azaro, Advisory and Consulting: Amcure GmbH and Orion Corporation. C Massard, Advisory and Consulting: Amgen, Astellas, AstraZeneca, Bayer, BeiGene, BMS, Celgene, Debiopharm, Genentech, Ipsen, Janssen, Lilly, MedImmune, MSD, Novartis, Pfizer, Roche, Sanofi, Orion; Principal/Sub-Investigator of Clinical Trials: Abbvie, Aduro, Agios, Amgen, Argen-x, Astex, AstraZeneca, Aveo pharmaceuticals, Bayer, Beigene, Blueprint, BMS, Boeringer Ingelheim, Celgene, Chugai, Clovis, Daiichi Sankyo, Debiopharm, Eisai, Eos, Exelixis, Forma, Gamamabs, Genentech, Gortec, GSK, H3 biomedecine, Incyte, Innate Pharma, Janssen, Kura Oncology, Kyowa, Lilly, Loxo, Lysarc, Lytix Biopharma, Medimmune, Menarini, Merus, MSD, Nanobiotix, Nektar Therapeutics, Novartis, Octimet, Oncoethix, Oncopeptides AB, Orion, Pfizer, Pharmamar, Pierre Fabre, Roche, Sanofi, Servier, Sierra Oncology, Taiho, Takeda, Tesaro, and Xencor, outside of submitted work. PA Cassier, received honoraria from Amgen, AstraZeneca, Blueprint Medicines, Novartis, Roche/Genentech, and Merck Serono; Investigator/Sub-Investigator of Clinical Trials: Abbvie, AstraZeneca, Bayer, Blueprint Medicines, Bristol-Myers Squibb, Celgene, Eli Lilly and Company, Glaxo Smith Kline, Innate Pharma, Janssen, Merck Serono, Merck Sharp Dohme, Novartis, Plexxikon, Genetech/Roche, Taiho Pharmaceuticals, and Transgene, outside of submitted work; Travel Accommodations and Expenses: Amgen, Bristol-Myers Squibb, Merck Sharp and Dohme, Netris Pharma, Novartis, and Roche. S Pant, Advisory and Consulting: Tyme, Inc., 4D-Pharma, Xencor, Ipsen; Principal/Sub-Investigator for Clinical Trials: Arcus, Arqule, Bristol-Myers Squibb, Eli Lilly, Five Prime Therapeutics, Glaxo Smith Kline, Holy Stone Healthcare Co., Tyme, Inc., Ipsen, Mirati Therapeutics, Inc., Novartis, Onco Response, Red Hill Biopharma, Ltd., Rgenix, Sanofi-Aventis, Xencor, Astellas, and Janssen, outside of submitted work. B Anderson, E Yuen, D Yu, G Oakley III, and KA Benhadji are current or past employees of Eli Lilly and Company and may hold company stocks. B Anderson is an employee of PRA Health Sciences and KA Benhadji is an employee of Taiho Oncology, and may hold company stocks.
Ethics approval
The study was approved by independent ethics committees or institutional review boards at each site. The study was registered at ClinicalTrials.gov as NCT02784795.
Informed consent
All patients provided written informed consent before enrollment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Massard, C., Cassier, P.A., Azaro, A. et al. A phase 1b study of crenigacestat (LY3039478) in combination with gemcitabine and cisplatin or gemcitabine and carboplatin in patients with advanced or metastatic solid tumors. Cancer Chemother Pharmacol 90, 335–344 (2022). https://doi.org/10.1007/s00280-022-04461-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-022-04461-z