Abstract
Purpose
Tepotinib is a highly selective, potent, mesenchymal–epithelial transition factor (MET) inhibitor, approved for the treatment of non-small cell lung cancer (NSCLC) harboring MET exon 14 skipping. Objectives of this population pharmacokinetic (PK) analysis were to evaluate the dose–exposure relationship of tepotinib and its major circulating metabolite, MSC2571109A, and to identify the intrinsic/extrinsic factors that are predictive of PK variability.
Methods
Data were included from 12 studies in patients with cancer and in healthy participants. A sequential modeling approach was used to analyze the parent and metabolite data, including covariate analyses. Potential associations between observed covariates and PK parameters were illustrated using bootstrap analysis-based forest plots.
Results
A two-compartment model with sequential zero- and first-order absorption, and a first-order elimination from the central compartment, best described the plasma PK of tepotinib in humans across the dose range of 30–1400 mg. The bioavailability of tepotinib was shown to be dose dependent, although bioavailability decreased primarily at doses above the therapeutic dose of 500 mg. The intrinsic factors of race, age, sex, body weight, mild/moderate hepatic impairment and mild/moderate renal impairment, along with the extrinsic factors of opioid analgesic and gefitinib intake, had no relevant effect on tepotinib PK. Tepotinib has a long effective half-life of ~ 32 h.
Conclusions
Tepotinib shows dose proportionality up to at least the therapeutic dose, and time-independent clearance with a profile appropriate for once-daily dosing. None of the covariates identified had a clinically meaningful effect on tepotinib exposure or required dose adjustments.
Similar content being viewed by others
Introduction
Tepotinib is a highly selective, potent, orally available, reversible, adenosine triphosphate competitive, small molecule mesenchymal–epithelial transition factor (MET) inhibitor, which has been approved in Argentina, Australia, Brazil, Canada, Europe (EU member states, as well as Liechtenstein, Iceland and Norway), Great Britain, Hong Kong, Israel, Japan, Korea, Singapore, Switzerland, Taiwan, and the US for the treatment of non-small cell lung cancer (NSCLC) harboring MET exon 14 skipping [1,2,3,4,5,6]; it is currently undergoing health authority reviews in multiple other countries and regions. The Japanese approval was the first globally for a MET inhibitor [5].
Tepotinib approval is supported by the results of the phase 2 study of tepotinib in patients with NSCLC and confirmed exon 14 skipping mutations (VISION, NCT02864992) [7]. Patients received oral tepotinib 500 mg once daily (QD) over 21-day cycles, and the primary endpoint was the objective response [7]. Tepotinib, administered as monotherapy in the VISION study, was associated with an acceptable and manageable safety profile.
During clinical development, tepotinib was assessed in patients with cancer at doses of 30–1400 mg QD [8,9,10]. Studies were designed to assess the maximum tolerated dose, recommended phase 2 dose, to compare formulations, and to assess the effects of intrinsic and extrinsic factors [8, 11, 12]. Several different capsule formulations (CFs) and tablet formulations (TFs) have been used during clinical development, aimed at increasing bioavailability and reducing pharmacokinetic (PK) variability. The initial CF formulation, CF1, contained non-micronized material, whereas all subsequent CFs and TFs used micronized material. CF1 was only used for part of the first-in-human study, with doses up to 230 mg [8]. This study also investigated another capsule formulation, CF2, at doses up to 1400 mg, as well as a tablet formation, TF1, at 500 and 1000 mg. The VISION study was conducted with TF2, another micronized tablet formulation with a dose of 500 mg (450 mg active moiety) [7]. The commercial formulation is TF3, a micronized tablet formulation with a dose strength of 250 mg, which has shown bioequivalence to TF2 (manuscript in preparation).
Tepotinib is absorbed after oral administration with a time to maximum concentration (Tmax) at steady state under fed conditions of approximately 8 h, and a terminal elimination half-life of approximately 32 h [1]. Tepotinib is a low-solubility drug [13] but has a high absolute bioavailability of 72% [14]. It is extensively cleared by the liver, predominantly via biliary clearance of unchanged drug, but is also metabolized via multiple pathways. Based on in vitro studies and human mass balance data, no metabolic pathway is dominant, and no pathway comprises more than 25% of the administered dose. Therefore, the interaction potential with co-administered drugs that interact with drug-metabolizing enzymes is considered to be low. The major circulating metabolite is MSC2571109A (R-enantiomer of M506), which contributed 41% of total circulating radioactivity (with tepotinib contributing 55% of the total radioactivity). Negligible amounts of this metabolite were excreted [14]. MSC2571109A is not thought to contribute to the efficacy of tepotinib, based on preclinical PK/efficacy and clinical PK profiling [15].
The objectives of this population PK analysis were to evaluate the dose–exposure relationship of tepotinib and its major circulating metabolite, MSC2571109A, in the overall population, and to identify the intrinsic and extrinsic factors that are predictive of PK variability.
Methods
Analysis set
The population PK analysis was based on data collected from 12 studies: five completed studies in patients with cancer, six completed studies in healthy participants, and the pivotal study (VISION) in patients with NSCLC harboring MET exon 14 skipping that was ongoing at the time of the analysis. An overview of these studies is provided in Table 1.
All tepotinib and metabolite concentrations were quantified using validated LC–MS/MS methods with lower limits of quantitation (LLOQ) of 5 ng/mL (0.168 ng/mL for studies 001 and 002), and 0.5 ng/mL for tepotinib and MSC2571109A, respectively.
All patients with at least one PK observation above the LLOQ were included in the analysis. Data below the LLOQ were excluded.
Population PK base model development
A sequential modeling approach was applied to analyze the parent and metabolite data. The tepotinib model was developed first, and the final tepotinib model was extended to characterize the MSC2571109A data, while keeping the individual parameters of the tepotinib model fixed.
Informed by graphical analysis, the following structural and statistical models were evaluated for tepotinib and the metabolite: absorption models for tepotinib, including first-order and sequential zero- and first-order absorption models, and disposition models for tepotinib and MSC2571109A, including one- and two-compartmental disposition models with first-order elimination from the central compartments. Interindividual random variability (IIV) was added to all relevant PK parameters, according to a lognormal distribution with standard deviation ω.
Covariate model development
A stepwise approach was utilized where an initial structural covariate model was established from the tepotinib base model, followed by a broader scope of covariate searching. Structural covariates per design that were known to impact the absorption of tepotinib were included into the base model after confirmation by graphical exploration and testing their relationship to the absorption-related parameters in a stepwise covariate model building procedure (SCM). This step included food status, formulation, and oral dose level.
In the second step, the remaining covariates were further evaluated, including demographics (age, body weight, race, and sex), patient characteristics (disease status, i.e., patient or healthy participant, tumor type, National Cancer Institute Organ Dysfunction Working Group [NCI-ODWG] classification of liver impairment [16], and baseline tumor burden), laboratory values (serum albumin, total bilirubin, alkaline phosphatase, international normalized ratio, and total protein), and co-medications (opioid analgesics and gefitinib). Since the phase 1 cross-over study 0039 did not show a relevant effect of concomitant omeprazole administration [11], co-administration of proton pump inhibitors was not investigated as a covariate in the PopPK analysis.
Tumor types included hepatocellular carcinoma (HCC), NSCLC, renal cell carcinoma, gastroesophageal cancer, colorectal cancer, and the remaining cancer categories (head/neck, breast, prostate, pancreas, and other solid tumors) combined.
The oral dose level was tested for its effect on apparent clearance (CL) of tepotinib. Estimated glomerular filtration rate (eGFR; calculated using the Modification of Diet in Renal Disease [MDRD] method [17]) was tested for its effect on apparent CL of tepotinib and MSC2571109A. The remaining covariates were tested for their effects on all PK parameters of tepotinib and MSC2571109A. The baseline value was assessed for most of the covariates, except for co-medication with μ-opioids, oral dose level and formulation, which were evaluated as time-varying covariates. Missing covariates were imputed with the population median value or most common value for continuous and categorical covariates, respectively. Disease-related covariates such as tumor size at baseline, were imputed with 0 for healthy participants and the median value among patients for patients.
SCM was applied in the structural covariate model building step, followed by an adaptive scope reduction (ASR) SCM in the following covariate searching step to reduce run times [18]. For the SCM procedures, the forward selection p value was set to 0.01, and the backward elimination p value to 0.001. The ASR threshold p value was set to the same as the forward p value of 0.01.
Continuous covariate relationships were coded as power models (Formula 1), and categorical covariates were coded as a fractional difference to the most common category (Formula 2). An alternative ‘hockey-stick’ parameterization (Formula 3) was tested for dose as continuous covariate on bioavailability of the parent drug (Par).
ParCovm is covariate m (Covm) for the parent drug and Covref is a reference covariate value for Covm, to which the covariate model is normalized (usually the median or mode).
The IIV covariance structure and residual unexplained variability (RUV) terms were re-assessed to establish the final covariate model.
Model selection and qualification
Model selection and qualification was based on the changes in the objective function value (OFV) provided by NONMEM and the visual inspection of graphical diagnostics, including goodness-of-fit plots and visual predictive checks (VPCs). For a more complicated model to be retained, it had to provide a significant improvement over the contending model (p < 0.05 hierarchical models), and provide plausible parameter estimates not associated with excessively high relative standard errors. It also had to demonstrate improvement in the graphical diagnostics and not result in a high (> 1000) condition number of the correlation matrix, indicating model instability [19].
The population PK analyses were performed using NONMEM version 7.3.0. Data management and further processing of NONMEM output were performed using R version 3.3.3. VPCs and covariate model building were run on PsN version 4.4.8.
Forest plot
The association or lack of association between the observed covariates and model-derived secondary PK parameters [e.g., area under the concentration–time curve over the dosing interval at steady state (AUCτ,ss)] for patients with cancer was illustrated using bootstrap analysis-based forest plots (see ESM 1 for method details). In the bootstrap analysis, N = 100 new data sets were generated with replacement from the analysis data set. The sampling was stratified by study, and the final models were fit to each of the resampled data sets. In the bootstrap for MSC2571109A, the parameter estimation was conditioned on the individual parameter estimates from the final tepotinib model. For categorical covariates, the mean and its 5th and 95th percentiles of PK parameters for each category were computed. For continuous covariates, the mean and its 5th and 95th percentiles of PK parameters, corresponding to the 0–5th and 95–100th percentile of the covariate, were computed. The impact of the covariates in the forest plots were presented on a relative scale. The reference value was the arithmetic mean of the corresponding PK parameter.
Results
Summary of analysis data
A total of 10,788 tepotinib concentrations from 613 study participants and 7197 MSC2571109A concentrations from 464 participants were included in the analysis. Key characteristics of the tepotinib and MSC2571109A analysis data sets are provided in Table 2. Concentrations of MSC2571109A were only measured after its discovery in the mass balance study [13], while the clinical development was ongoing; hence, there are fewer observation records available for MSC2571109A than tepotinib.
The analysis included 591 participants with an evaluable baseline eGFR (mean 99.8 mL/min/1.73 m2, standard deviation 26.2 mL/min/1.73 m2, range 39.4 to 236 mL/min/1.73 m2), of which 24 were classified as having moderate renal impairment (eGFR ≥ 30 and < 60 mL/min/1.73 m2) and contributed 390 tepotinib PK observations (3.6% to the total data set).
A total of 146 participants were classed as having mild hepatic impairment (contributing 18.3% of the total number of tepotinib PK observations) and 16 were classed as having moderate hepatic impairment (contributing 1.5% of the total number of tepotinib PK observations), according to the NCI-ODWG criteria for hepatic dysfunction. Only three participants were classed as having severe hepatic impairment (contributing 0.6% of the total number of tepotinib PK observations).
The fraction of participants with missing covariate values ranged between 0 and 18%. The three covariates with the largest fraction of missing values were hepatitis B virus/hepatitis C virus (HBV/HCV) status at screening, serum-albumin and total protein at baseline, with 18%, 17% and 13% missing values, respectively.
Tepotinib population PK model
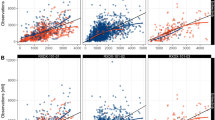
A two-compartment model with sequential zero- and first-order absorption, and a first-order elimination from the central compartment, with an additive residual error on the log scale, best described the plasma PK of tepotinib in humans across the dose range of 30–1400 mg (Fig. 1). The IIV terms are included on CLpar, first-order absorption rate constant (ka), zero-order absorption duration (D1), and bioavailability of parent drug (Fpar).
Illustration of the base population pharmacokinetics model for tepotinib and MSC2571109A. CL clearance, D1 zero-order absorption duration, FM fraction of tepotinib metabolized to MSC2571109A, ka first-order absorption rate constant, met metabolite, par parent, Q inter-compartmental clearance, Vc central volume of distribution, Vp peripheral volume of distribution
Apparent CL for tepotinib was estimated at 20.4 L/h, with an IIV of 33.5%. The relative absorption bioavailability was fixed to 1, with an estimated IIV of 28.3%. Tepotinib has a long effective half-life of ~ 32 h.
Several covariates were found to have a statistically significant influence on tepotinib PK parameters based on the ASR SCM procedure; all are listed in Table 3. Tepotinib exposure was significantly reduced after administration of CF1 (65.6% reduction of Fpar, 44.2% reduction of ka), and after fasting (20.9% reduction of Fpar, 56.1% reduction of ka), compared with a typical participant taking TF2 with a non-high-fat meal.
The bioavailability of tepotinib was shown to be dose-dependent, with decreasing bioavailability at high doses (Fig. 2). The model-predicted effect of tepotinib AUCτ,ss is illustrated in Fig. 2 and indicates a lack of relevant deviation from dose linearity at tepotinib doses up to 500 mg. However, relative bioavailability for the supratherapeutic tepotinib dose of 1000 mg is 0.79 compared with the Fpar for a dose of 500 mg.
Predicted tepotinib AUCss versus dose, with and without the estimated dose effect on Fpar. The blue line represents the relationship between AUCss and dose according to the final tepotinib model, while the grey, dashed line displays the theoretical relationship between tepotinib AUCss and dose if the dose had no impact on Fpar. AUCss area under the curve at steady state, par parent, eGFR estimated glomerular filtration rate, INR international normalized ratio, NSCLC non-small cell lung cancer, QD once daily, TF tablet formulation. Note: The prediction is for a typical patient with NSCLC (59 years, 72 kg, serum albumin = 40 g/L, eGFR = 97.28 mL/min/1.73 m2, INR = 1.06) receiving 500 mg QD tepotinib TF3 with food
Liver dysfunction (defined by NCI-ODWG) slightly reduced tepotinib bioavailability (7.29%) after oral administration, causing decreased total exposure in participants with liver dysfunction or cirrhosis. The apparent CL of tepotinib was found to be positively correlated to eGFR, but with a seemingly limited influence on exposure (eGFRs of 30, 60, 80 to 110 mL/min/1.73 m2 for a typical individual receiving 500 mg of tepotinib led to CLpar values of 16.2, 18.6, 19.7 and 20.9 L/h, respectively). Intake of opioid analgesics caused a 16.7% reduction in apparent CL of tepotinib, but the influence on tepotinib AUCτ,ss also appeared to be limited. No statistically significant influence of gefitinib co-administration on tepotinib PK was identified. Refer to ESM 12 for observed vs. predicted tepotinib plasma concentrations. The equations for the final pop PK model are available in ESM 13.
MSC2571109A population PK model
The plasma PK of MSC2571109A was best described by a two-compartment model that includes input from the central compartment of the tepotinib model, scaled by fraction of tepotinib metabolized to MSC2571109A and a first-order elimination from the central compartment.
The fraction metabolized (FM) is unknown and unidentifiable from the current data, thus this parameter was fixed to 1 but the associated IIV was estimated. The IIV terms are also included on apparent CL of metabolite (CLmet), central volume of metabolite distribution (Vc,met), inter-compartmental metabolite clearance (Qmet) and peripheral volume of metabolite distribution (Vp,met).
In the covariate modeling step, several covariates were identified to significantly influence MSC2571109A PK parameters (Table 3). Apparent CL was positively correlated with eGFR and negatively correlated with body weight.
Values of derived secondary PK parameters for patients with cancer receiving the clinical dose of tepotinib TF3 500 mg/day with food or a reduced dose level of 250 mg/day were also estimated using a bootstrap analysis of the final population model (see ESM 2).
Model qualification
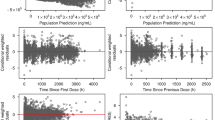
Goodness-of-fit plots for the final models of tepotinib (ESM 3 and ESM 4) and MSC2571109A (ESM 5 and ESM 6) indicated that these final models well described the plasma concentrations of both the parent and metabolite. Tepotinib PK appeared to be time-independent, i.e., the PK after a single dose was predictive of the PK at steady state.
The prediction-corrected VPC showed a strong agreement for the observed and model-predicted median PK profiles. Plots of the individual random effects on tepotinib apparent CL (ESM 7 and ESM 8) and bioavailability (ESM 9 and ESM 10), estimated with the base and final models and plotted against individual covariates, confirm that including covariates in the tepotinib model removed the trend in the random effect versus covariates observed with the base model. Model diagnostic plots demonstrated a strong model performance, supporting its usability to derive individual predictions of exposure.
Model-based simulation and forest plots
In Fig. 3, the model-predicted time profile of tepotinib plasma concentration is shown for a typical patient with NSCLC (59 years, 72 kg, serum albumin = 40 g/L, eGFR = 97.28 mL/min/1.73 m2, INR = 1.06) receiving the clinical dose of 500 mg/day tepotinib TF3 with food.
Simulation of tepotinib PK profile for a typical patient with NSCLC (59 years, 72 kg, serum albumin = 40 g/L, eGFR = 97.28 mL/min/1.73 m2, INR = 1.06) receiving 500 mg QD tepotinib TF3 with food. The solid black line represents the median prediction of the PK time profile, and the green shaded area represents a simulation-based 5–95% prediction interval for PK time profile. eGFR estimated glomerular filtration rate, INR international normalized ratio, NSCLC non-small cell lung cancer, PK pharmacokinetics, QD once daily, TF tablet formulation
The population PK analysis did not find ethnicity to be a statistically significant covariate on the exposure of tepotinib. This lack of influence was further investigated with boxplots comparing model-predicted AUCτ,ss after 500 mg QD dosing for different ethnicity categories (Fig. 4), where the individual predicted AUCτ,ss values also showed no trend.
The distribution of tepotinib AUCss stratified by selected race categories, based on the final tepotinib population PK model and using the analysis data set. The predictions of AUCτ,ss are for Caucasian, Other East Asian and Japanese participants in the tepotinib analysis data set receiving 500 mg tepotinib with the TF1 or TF2 formulation and having a standard breakfast. The horizontal line in the box indicates the median value, the box edges represent the 25th and 75th percentiles, and the whiskers extend from the box to the furthest data points still within a distance of 1.5 times the interquartile range from the box. Data points, which are jittered in the horizontal direction, show the individually predicted AUCss values. The numbers represent the number of individuals in each strata. AUCss area under the curve at steady state, PK pharmacokinetics, TF tablet formulation
The influence of covariates on tepotinib and MSC2571109A AUCτ,ss is illustrated in Fig. 5 and ESM 11. Most covariates showed no or marginal effects on tepotinib AUC with 90% CIs within the 80–125% range. These included race, hepatic impairment, renal impairment, age, sex, body weight, and intake of opioid analgesics or gefitinib. Exceptions included formulation, food intake status and tumor type of renal cell carcinoma.
Forest plot showing the association of the predicted tepotinib AUCss and covariates assuming a dosing regimen of 500 mg tepotinib daily, based on the final tepotinib population PK model, for cancer patients in the analysis data set. The closed symbols represent the mean ratio of individual parameter estimates for the applicable covariate category or value (5th or 95th percentile for continuous covariates) percentile relative to the mean parameter estimate (vertical solid line) for cancer patients in the analysis dataset. The whiskers represent the 90% CI of the mean values, based on 100 bootstrap samples. AUCss area under the curve at steady state, CI confidence interval, ECOG Eastern Cooperative Oncology Group, eGFR estimated glomerular filtration rate, NCI-ODG National Cancer Institute Organ Dysfunction Group, PK pharmacokinetic
The mean effect on MSC2571109A AUCτ,ss mostly remained within the range of 80–125% for the typical patient with cancer in the analysis data set for all covariates, with the exception of moderate renal impairment, formulation, food intake status and tumor types (ESM 11). Given the negligible contribution of MSC2571109A to the clinical efficacy of tepotinib, it is unlikely that these covariate effects are clinically relevant.
Discussion
Population PK modeling of tepotinib and MSC2571109A provides comprehensive model-based analytical tools to integrate both parent and metabolite PK profiles of 613 participants from 12 studies. These participants were administered four different formulations of tepotinib across a broad dose range of 30–1400 mg/day. These data have been well fitted to a two-compartment model with sequential zero- and first-order absorption and a first-order elimination from the central compartment for tepotinib, as well as a two-compartment model with input from the central compartment in the tepotinib model, scaled by FM and a first-order elimination from the central compartment for MSC2571109A. Since FM was unknown and could not be estimated from the data, it was fixed to 1 while the metabolite model was developed conditionally on the (fixed) tepotinib model. The fixed FM will only affect the numerical values of the primary metabolite PK parameters (CL and V) while the predicted metabolite concentrations and derived secondary parameters are unaffected. The model-predicted PK parameters are in agreement with the non-compartmental analyses of data from reported studies with tepotinib [8, 10, 14].
Tepotinib showed moderate PK IIV (33.5% for CL and 28.3% for bioavailability). No direct estimate of the intra-individual variability in tepotinib CL was obtained in the population PK analysis, but, given that the residual variability was 33.7% (which also includes variability due to the bioanalytical assay, potential model misspecification and any errors in correct documentation of PK sample timing), it may be concluded that the intra-individual variability in tepotinib PK is less than the IIV.
Tepotinib PK are approximately dose-proportional up to the therapeutic dose of 500 mg. At higher doses, tepotinib PK was shown to increase sub-proportionally to doses with limited exposure increases (less than a doubling) seen between 500 and 1400 mg. This finding should be interpreted with caution as most data for doses beyond 500 mg were obtained with the CF2 formulation in Study 001.
Tepotinib exhibits time-independent PK and has a long effective half-life of ~ 32 h with small peak-trough fluctuations, all of which support QD dosing. This differentiates tepotinib from other MET inhibitors, such as capmatinib, that require twice-daily dosing [20].
Effect of intrinsic factors on tepotinib PK
Renal impairment
Mild and moderate renal impairment has been shown to have no clinically relevant effects on tepotinib PK (based on data from patients enrolled in the VISION study). In the covariate analysis, renal function was assessed using eGFR as determined by the MDRD formula. This metric was preferred over Cockcroft–Gault-predicted creatinine clearance as eGFR is independent of body weight and potentially leads to lower multi-collinearity with body mass covariates and less selection bias in the SCM procedure. The forest plot suggested that the apparent clearance of tepotinib did not show clinically meaningful association with eGFR: AUCτ,ss was 2% higher in patients with moderate renal impairment (39.4 mL/min/1.73 m2 ≤ eGFR ≤ 59 mL/min/1.73 m2) in comparison to the mean observed eGFR value of 99.8 mL/min/1.73 m2. As expected, renal excretion minimally contributes to the total elimination: in the mass balance study, the majority (77.9%) of the total radiolabeled material was excreted via feces [14]. Urinary excretion was identified as a minor route with a recovery of only 13.6%. Both of these findings indicate that dose adjustment of tepotinib is not required for mild/moderate renal impairment.
Hepatic impairment
The effect of reduced liver function on Fpar translates into lower total tepotinib AUC in participants with mild to moderate hepatic impairment (HI, based on NCI-ODWG classification), albeit to a not clinically relevant extent (90% CI for the mean ratio within the 80–125% interval, refer to Fig. 5). The effect of HI on exposure was specifically investigated in a hepatic impairment study (Study 0028) in which participants with moderate HI had 13% lower total plasma AUC, as determined by non-compartmental analysis, than healthy participants. However, in the same study, free tepotinib concentrations were similar in all hepatic function groups (manuscript in preparation).
Given the lack of clinically relevant changes in tepotinib free exposure and the flat exposure–response relationship over the exposure range achieved with the clinical dose of 500 mg/day [21], no loss of efficacy or elevated risk of adverse events is anticipated with mild/moderate hepatic impairment. Dose adjustment of tepotinib is not required for mild/moderate hepatic impairment, and there is insufficient evidence to draw conclusions with regard to severe hepatic impairment.
Ethnicity
Covariate model analysis and simulation-based comparisons suggested no impact of ethnicity on tepotinib PK. This finding reinforces the rationale of the VISION study as a multi-regional clinical trial (MRCT; conducted in the US, EU, Japan, China and other Asian countries) following ICH E17 principles [22], which could be accepted by regulatory authorities across regions and countries as the primary source of evidence to support marketing approval. This pivotal MRCT and the lack of ethnicity effect shown from the current modeling were important pillars in the first global approval in Japan.
Other intrinsic factors
There were no clinically relevant effects of age (> 18 years), sex, or body weight, hence no dose adjustments are required on the basis of any of these factors and a flat-dose regimen is supported by the analysis. Tumor type did not appear to influence tepotinib PK, and although lower exposures were seen in patients with renal cell carcinoma, this result must be considered in light of the low sample size (N = 5) for this patient group and the impact of other confounding factors.
Extrinsic factors
Food and formulation
Tepotinib has been administered as several CFs and TFs during development. The CF1 formulation was the only one to use a non-micronized drug substance and was only used for the initial doses (up to 230 mg) in the first-in-human study [8]. Micronization of the drug substance in all other tested formulations was shown to increase tepotinib bioavailability, presumably via increased solubility leading to increased absorption. Likewise, the presence of food has been shown to increase tepotinib exposure due to increased in vivo solubility in the fed state. Studies in healthy participants confirmed these findings, showing an approximate two-fold increase in tepotinib exposure in the presence of food (high-fat meal) [11]. Tepotinib is, therefore, recommended to be administered with food to increase its bioavailability, and the phase 2 program, including the pivotal VISION trial, was conducted accordingly.
Co-administration with opioids or gefitinib
Opioid analgesics were included as continuous covariates based on the ability of µ-opioid receptor agonists to influence gastrointestinal motility. Twenty-four percent of the tepotinib observations were associated with concomitant administration of opioid analgesics. However, the observed effect of concomitant opioid use on CL/F did not lead to clinically meaningful changes in AUC.
Although, based on its ADME properties, no interaction with the EGFR inhibitor gefitinib was expected, concomitant administration of gefitinib was included as a covariate as patients in Study 006 received both drugs. Co-administration of tepotinib with gefitinib had no effect on tepotinib or gefitinib exposure [9].
Conclusions
The PK of tepotinib and MSC2571109A were both well described using a two-compartment model with first-order elimination. Tepotinib has dose-dependent bioavailability above 500 mg and time-independent CL, with a profile appropriate for QD dosing.
The intrinsic factors of race, age, sex, body weight, mild/moderate hepatic impairment, and mild/moderate renal impairment, along with the extrinsic factors of opioid analgesic and gefitinib intake, had no relevant effect on tepotinib PK. Accordingly, no dose adjustment is required for any of these factors.
Data availability
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to Merck Healthcare KGaA, Darmstadt, Germany, Data Sharing Policy. All requests should be submitted in writing to Merck Healthcare KGaA, Darmstadt, Germany, data sharing portal, which can be found at https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html. When Merck Healthcare KGaA, Darmstadt, Germany has a co-research, co-development, or co-marketing or co-promotional agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, Merck Healthcare KGaA, Darmstadt, Germany will endeavor to gain agreement to share data in response to requests.
References
FDA (2021) TEPMETKO (tepotinib) US Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214096s000lbl.pdf. Accessed Nov 2021
Bladt F, Faden B, Friese-Hamim M et al (2013) EMD 1214063 and EMD 1204831 constitute a new class of potent and highly selective c-Met inhibitors. Clin Cancer Res 19(11):2941–2951. https://doi.org/10.1158/1078-0432.ccr-12-3247
Anvisa (2021) Tepotinib approval (Brazil). https://www.gov.br/anvisa/pt-br/composicao/diretoria-colegiada/reunioes-da-diretoria/votos/2020/rop-13.2020/item-2-4-3-voto-161-dire4.pdf/view. Accessed Jul 2021
Government of Canada (2021) Qualifying Notice for Tepmetko. https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/notice-compliance/conditions/qualifying-notice-tepmetko-242300.html. Accessed Jul 2021
PMDA (2020) TEPMETKO approval in Japan. https://www.pmda.go.jp/files/000236338.pdf. Accessed Jul 2021
SwissMedic (2021) TEPMETKO approval (Switzerland). https://www.swissmedic.ch/swissmedic/en/home/humanarzneimittel/authorisations/new-medicines/tepmetko-filmtabletten-tepotinibum.html. Accessed Jul 2021
Paik P, Felip E, Veillon R et al (2020) Tepotinib in non-small-cell lung cancer with MET exon 14 skipping mutations. N Engl J Med 383(10):931–943. https://doi.org/10.1056/NEJMoa2004407
Falchook GS, Kurzrock R, Amin HM et al (2020) First-in-man phase I trial of the selective MET inhibitor tepotinib in patients with advanced solid tumors. Clin Cancer Res 26(6):1237–1246. https://doi.org/10.1158/1078-0432.CCR-19-2860
Wu YL, Cheng Y, Zhou J et al (2020) Tepotinib plus gefitinib in patients with EGFR-mutant non-small-cell lung cancer with MET overexpression or MET amplification and acquired resistance to previous EGFR inhibitor (INSIGHT study): an open-label, phase 1b/2, multicentre, randomised trial. Lancet Respir Med 8(11):1132–1143. https://doi.org/10.1016/S2213-2600(20)30154-5
Shitara K, Yamazaki K, Tsushima T et al (2020) Phase I trial of the MET inhibitor tepotinib in Japanese patients with solid tumors. Jpn J Clin Oncol 50(8):859–866. https://doi.org/10.1093/jjco/hyaa042
Strotmann R, Heuer J, Becker A, Krebs-Brown A, Mammasse N, Yalkinoglu Ö (2019) Bioavailability of tepotinib: impact of omeprazole and food. Ann Oncol 30(Suppl. 5):v182
Yalkinoglu Ö, Heuer J, Becker A, Krebs-Brown A, Strotmann R (2019) Drug-drug interaction profile of tepotinib with CYP3A and P-gp substrates. Ann Oncol 30(Suppl. 5):v182
Fink C, Lecomte M, Badolo L, Wagner K, Mäder K, Peters SA (2020) Identification of solubility-limited absorption of oral anticancer drugs using PBPK modeling based on rat PK and its relevance to human. Eur J Pharm Sci. https://doi.org/10.1016/j.ejps.2020.105431
Johne A, Scheible H, Becker A, van Lier JJ, Wolna P, Meyring M (2020) Open-label, single-center, phase I trial to investigate the mass balance and absolute bioavailability of the highly selective oral MET inhibitor tepotinib in healthy volunteers. Invest New Drugs 38(5):1507–1519. https://doi.org/10.1007/s10637-020-00926-1
Xiong W, Friese-Hamim M, Johne A et al (2021) Translational pharmacokinetic-pharmacodynamic modeling of preclinical and clinical data of the oral MET inhibitor tepotinib to determine the recommended phase II dose. CPT Pharmacometrics Syst Pharmacol 10(5):428–440. https://doi.org/10.1002/psp4.12602
Patel H, Egorin MJ, Remick SC et al (2004) Comparison of Child-Pugh (CP) criteria and NCI organ dysfunction working group (NCI-ODWG) criteria for hepatic dysfunction (HD): implications for chemotherapy dosing. J Clin Oncol 22(14_suppl):6051–6051. https://doi.org/10.1200/jco.2004.22.90140.6051
Hunsicker L, Adler S, Caggiula A et al (1997) Predictors of the progression of renal disease in the modification of diet in renal disease study. Kidney Int 51(6):1908–1919. https://doi.org/10.1038/KI.1997.260
Jonsson EN, Harling K (2018) Increasing the efficiency of the covariate search algorithm in the SCM. Presented at PAGE, Athens, Greece, https://www.page-meeting.org/?abstract=8429 Accessed Jul 2021
Mould D, Upton R (2013) Basic concepts in population modeling, simulation, and model-based drug development-part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacometrics Syst Pharmacol 2(4):38. https://doi.org/10.1038/psp.2013.14
Wolf J, Seto T, Han J-YY et al (2020) Capmatinib in MET exon 14–mutated or MET–amplified non-small-cell lung cancer. N Engl J Med 383(10):944–957. https://doi.org/10.1056/nejmoa2002787
Paik P, Xiong W, Hietala SF et al (2020) 584P Tepotinib exposure–response analyses of safety and efficacy in patients with solid tumours. Ann Oncol 31:S494–S495. https://doi.org/10.1016/j.annonc.2020.08.698
EMA (2017) ICH guideline E17 on general principles for planning and design of multi-regional clinical trials. https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-e17-general-principles-planning-design-multi-regional-clinical-trials-step-5-first_en.pdf. Accessed Aug 2021
Wesolowski R, Zhao M, Geyer SM et al (2014) Phase I trial of the PARP inhibitor veliparib (V) in combination with carboplatin (C) in metastatic breast cancer (MBC). J Clin Oncol 32(15_suppl):1074. https://doi.org/10.1200/JCO.2014.32.15_SUPPL.1074
Decaens T, Barone C, Assenat E et al (2021) Phase 1b/2 trial of tepotinib in sorafenib pretreated advanced hepatocellular carcinoma with MET overexpression. Br J Cancer 125(2):190–199. https://doi.org/10.1038/s41416-021-01334-9
ClinicalTrials.gov (2018) c-Met Second-Line Hepatocellular Carcinoma. . Accessed Aug 2021
ClinicalTrials.gov (2019) Tepotinib Hepatic Impairment Trial. . Accessed Jul 2021
ClinicalTrials.gov (2018) Effect of a Proton Pump Inhibitor on the Pharmacokinetics (PK) of Tepotinib. . Accessed Aug 2021
ClinicalTrials.gov (2019) Bioequivalence of Tablet Formulation 3 (TF3) and Tablet Formulation 2 (TF2) and Effect of Food on the Pharmacokinetics (PK) of Tepotinib. . Accessed Aug 2021
Acknowledgements
The authors would like to thank Samantha Abel of Syneos Health Communications UK Ltd for medical writing assistance for this manuscript, which was funded by Merck Healthcare KGaA, Darmstadt, Germany.
Funding
The studies included in this article and the analyses reported were funded by Merck Healthcare KGaA, Darmstadt, Germany.
Author information
Authors and Affiliations
Contributions
Conceptualization: WX, RS, ENP, OP, PG. Data curation: WX, RS, ENP, OP, PG. Formal analysis: ENJ. Methodology: WX, RS, ENP, OP, PG. Project administration: WX, RS. Supervision: WX, RS. Validation: WX, RS. Visualization: WX, RS. Writing—original draft: WX. Writing—review and editing: All authors.
Corresponding author
Ethics declarations
Conflict of interest
Wenyuan Xiong was employed by the Merck Institute of Pharmacometrics, Lausanne, Switzerland, an affiliate of Merck KGaA for the duration of the study. Orestis Papasouliotis and Pascal Girard are employees of the Merck Institute of Pharmacometrics, Lausanne, Switzerland, an affiliate of Merck KGaA. Rainer Strotmann is an employee of Merck Healthcare KGaA, Darmstadt, Germany. E. Niclas Jonsson is an employee of Pharmetheus AB, Sweden.
Ethical approval
All studies were approved by appropriate ethics committees and were conducted in accordance with the principles of the Declaration of Helsinki and the International Council on Harmonisation Good Clinical Practice guidelines.
Consent to participate
Written informed consent was obtained from all participants before any study-related procedures were performed.
Consent for publication
All authors consented to the publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xiong, W., Papasouliotis, O., Jonsson, E.N. et al. Population pharmacokinetic analysis of tepotinib, an oral MET kinase inhibitor, including data from the VISION study. Cancer Chemother Pharmacol 89, 655–669 (2022). https://doi.org/10.1007/s00280-022-04423-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-022-04423-5