Abstract
Background
Osteosarcoma is a prevalent type of bone tumor in children and adolescents, with limited treatment and poor prognosis. Abemaciclib, an inhibitor of cyclin-dependent kinases 4 and 6 (CDK4/6), is approved for the treatment of advanced breast cancer as single agent therapy and is currently under investigation in clinical trials for the treatment of several solid tumors.
Methods
The efficacy of abemaciclib was determined using osteosarcoma cellular assays and xenograft mouse model. The combination studies were performed based on the Chou-Talalay method. Immunoblotting analysis was performed to determine the underlying mechanisms of abemaciclib in osteosarcoma cell lines.
Results
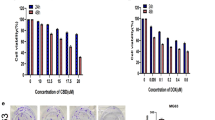
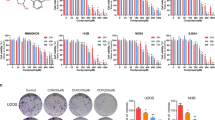
Abemaciclib potently inhibits growth, anchorage-independent colony formation and survival of a panel of osteosarcoma cell lines, with IC50 range from 90 nM to >20 μM. In addition, the combination of abemaciclib and doxorubicin is synergistic and antagonistic in abemaciclib-sensitive (IC50 <1 μM) and abemaciclib-resistant (IC50 >1 μM), respectively. Abemaciclib inhibits tumor formation and growth in a dose-dependent manner without causing significant drug toxicity in mice. The combination of abemaciclib and doxorubicin results in much greater efficacy than doxorubicin alone in inhibiting tumor growth throughout the whole treatment duration. Abemaciclib acts on osteosarcoma via suppressing CDK4/6-Cyclin D-Rb pathway.
Conclusions
Our pre-clinical evidence provides a rationale of initializing clinical trial of investigating the efficacy of abemaciclib in combination with doxorubicin in osteosarcoma patients. Our work also highlights the therapeutic value of CDK4/6 inhibition in osteosarcoma with proper function of Rb.







Similar content being viewed by others
Data availability
Data will be made available from the corresponding author upon reasonable request.
References
Sadykova LR, Ntekim AI, Muyangwa-Semenova M, Rutland CS, Jeyapalan JN, Blatt N, Rizvanov AA (2020) Epidemiology and risk factors of osteosarcoma. Cancer Invest 38:259–269
Yu D, Zhang S, Feng A, Xu D, Zhu Q, Mao Y, Zhao Y, Lv Y, Han C, Liu R, Tian Y (2019) Methotrexate, doxorubicin, and cisplatinum regimen is still the preferred option for osteosarcoma chemotherapy: a meta-analysis and clinical observation. Medicine (Baltimore). https://doi.org/10.1097/MD.0000000000015582
Kager L, Tamamyan G, Bielack S (2017) Novel insights and therapeutic interventions for pediatric osteosarcoma. Future Oncol 13:357–368
Jafari F, Javdansirat S, Sanaie S, Naseri A, Shamekh A, Rostamzadeh D, Dolati S (2020) Osteosarcoma: a comprehensive review of management and treatment strategies. Ann Diagn Pathol. https://doi.org/10.1016/j.anndiagpath.2020.151654
Kitajima S, Li F, Takahashi C (2020) Tumor milieu controlled by RB tumor suppressor. Int J Mol Sci. https://doi.org/10.3390/ijms21072450
Guzman F, Fazeli Y, Khuu M, Salcido K, Singh S, Benavente CA (2020) Retinoblastoma tumor suppressor protein roles in epigenetic regulation. Cancers (Basel). https://doi.org/10.3390/cancers12102807
Gao X, Leone GW, Wang H (2020) Cyclin D-CDK4/6 functions in cancer. Adv Cancer Res 148:147–169
VanArsdale T, Boshoff C, Arndt KT, Abraham RT (2015) Molecular pathways: targeting the cyclin D-CDK4/6 Axis for cancer treatment. Clin Cancer Res 21:2905–2910
Zhou Y, Shen JK, Yu Z, Hornicek FJ, Kan Q, Duan Z (1864) Expression and therapeutic implications of cyclin-dependent kinase 4 (CDK4) in osteosarcoma. Biochim Biophys Acta Mol Basis Dis 2018:1573–1582
Martin JM, Goldstein LJ (2018) Profile of abemaciclib and its potential in the treatment of breast cancer. Onco Targets Ther 11:5253–5259
Patnaik A, Rosen LS, Tolaney SM, Tolcher AW, Goldman JW, Gandhi L, Papadopoulos KP, Beeram M, Rasco DW, Hilton JF, Nasir A, Beckmann RP, Schade AE, Fulford AD, Nguyen TS, Martinez R, Kulanthaivel P, Li LQ, Frenzel M, Cronier DM, Chan EM, Flaherty KT, Wen PY, Shapiro GI (2016) Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer discov 6:740–753
Yadav B, Wennerberg K, Aittokallio T, Tang J (2015) Searching for drug synergy in complex dose-response landscapes using an interaction potency model. Comput Struct Biotechnol J 13:504–513
Borowicz S, Van Scoyk M, Avasarala S, Karuppusamy Rathinam MK, Tauler J, Bikkavilli RK, Winn RA (2014) The soft agar colony formation assay. J Vis Exp. https://doi.org/10.3791/51998
Liu ZQ, Mahmood T, Yang PC (2014) Western blot: technique, theory and trouble shooting. N Am J Med Sci 6:160
Tate SC, Sykes AK, Kulanthaivel P, Chan EM, Turner PK, Cronier DM (2018) A Population pharmacokinetic and pharmacodynamic analysis of abemaciclib in a phase I clinical trial in cancer patients. Clin Pharmacokinet 57:335–344
Montanaro L, Mazzini G, Barbieri S, Vici M, Nardi-Pantoli A, Govoni M, Donati G, Trere D, Derenzini M (2007) Different effects of ribosome biogenesis inhibition on cell proliferation in retinoblastoma protein- and p53-deficient and proficient human osteosarcoma cell lines. Cell Prolif 40:532–549
Dowless M, Lowery CD, Shackleford T, Renschler M, Stephens J, Flack R, Blosser W, Gupta S, Stewart J, Webster Y, Dempsey J, VanWye AB, Ebert P, Iversen P, Olsen JB, Gong X, Buchanan S, Houghton P, Stancato L (2018) Abemaciclib is active in preclinical models of Ewing sarcoma via multipronged regulation of cell cycle, DNA methylation, and interferon pathway signaling. Clin Cancer Res 24:6028–6039
Bollard J, Miguela V, Ruiz de Galarreta M, Venkatesh A, Bian CB, Roberto MP, Tovar V, Sia D, Molina-Sanchez P, Nguyen CB, Nakagawa S, Llovet JM, Hoshida Y, Lujambio A (2017) Palbociclib (PD-0332991), a selective CDK4/6 inhibitor, restricts tumour growth in preclinical models of hepatocellular carcinoma. Gut 66:1286–1296
Choi YJ, Anders L (2014) Signaling through cyclin D-dependent kinases. Oncogene 33:1890–1903
Sherr CJ, Beach D, Shapiro GI (2016) Targeting CDK4 and CDK6: from discovery to therapy. Cancer Discov 6:353–367
Braal CL, Jongbloed EM, Wilting SM, Mathijssen RHJ, Koolen SLW, Jager A (2020) Inhibiting CDK4/6 in breast cancer with palbociclib, ribociclib, and abemaciclib: similarities and differences. Drugs. https://doi.org/10.1007/s40265-020-01461-2
Oshiro H, Tome Y, Miyake K, Higuchi T, Sugisawa N, Kanaya F, Nishida K, Hoffman RM (2021) Combination of CDK4/6 and mTOR inhibitors suppressed doxorubicin-resistant osteosarcoma in a patient-derived orthotopic xenograft mouse model: a translatable strategy for recalcitrant disease. Anticancer Res 41:3287–3292
Francis AM, Alexander A, Liu Y, Vijayaraghavan S, Low KH, Yang D, Bui T, Somaiah N, Ravi V, Keyomarsi K, Hunt KK (2017) CDK4/6 Inhibitors sensitize Rb-positive sarcoma cells to wee1 kinase inhibition through reversible cell-cycle arrest. Mol Cancer Ther 16:1751–1764
Jin D, Tran N, Thomas N, Tran DD (2019) Combining CDK4/6 inhibitors ribociclib and palbociclib with cytotoxic agents does not enhance cytotoxicity. PLoS One. https://doi.org/10.1371/journal.pone.0223555
McClendon AK, Dean JL, Rivadeneira DB, Yu JE, Reed CA, Gao E, Farber JL, Force T, Koch WJ, Knudsen ES (2012) CDK4/6 inhibition antagonizes the cytotoxic response to anthracycline therapy. Cell Cycle 11:2747–2755
Naz S, Sowers A, Choudhuri R, Wissler M, Gamson J, Mathias A, Cook JA, Mitchell JB (2018) Abemaciclib, a selective CDK4/6 inhibitor, enhances the radiosensitivity of non-small cell lung cancer in vitro and in vivo. Clin Cancer Res 24:3994–4005
Nair AB, Jacob S (2016) A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7:27–31
Sritharan S, Sivalingam N (2021) A comprehensive review on time-tested anticancer drug doxorubicin. Life Sci. https://doi.org/10.1016/j.lfs.2021.119527
Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, Ginther C, Atefi M, Chen I, Fowst C, Los G, Slamon DJ (2009) PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res 11:R77
Logan JE, Mostofizadeh N, Desai AJ, Euw EVON, Conklin D, Konkankit V, Hamidi H, Eckardt M, Anderson L, Chen HW, Ginther C, Taschereau E, Bui PH, Christensen JG, Belldegrun AS, Slamon DJ, Kabbinavar FF (2013) PD-0332991, a potent and selective inhibitor of cyclin-dependent kinase 4/6, demonstrates inhibition of proliferation in renal cell carcinoma at nanomolar concentrations and molecular markers predict for sensitivity. Anticancer Res 33(8):2997–3004
Hamilton E, Infante JR (2016) Targeting CDK4/6 in patients with cancer. Cancer Treat Rev 45:129–138
Cen L, Carlson BL, Schroeder MA, Ostrem JL, Kitange GJ, Mladek AC, Fink SR, Decker PA, Wu W, Kim JS, Waldman T, Jenkins RB, Sarkaria JN (2012) p16-Cdk4-Rb axis controls sensitivity to a cyclin-dependent kinase inhibitor PD0332991 in glioblastoma xenograft cells. Neuro Oncol 14:870–881
Clark AS, Karasic TB, DeMichele A, Vaughn DJ, O’Hara M, Perini R, Zhang P, Lal P, Feldman M, Gallagher M, O’Dwyer PJ (2016) Palbociclib (PD0332991)-a selective and potent cyclin-dependent kinase inhibitor: a review of pharmacodynamics and clinical development. JAMA Oncol 2:253–260
Acknowledgements
This work was supported by a research grant provided by Hubei University of Medicine (20170616).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, D., Bao, H. Abemaciclib is synergistic with doxorubicin in osteosarcoma pre-clinical models via inhibition of CDK4/6–Cyclin D–Rb pathway. Cancer Chemother Pharmacol 89, 31–40 (2022). https://doi.org/10.1007/s00280-021-04363-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-021-04363-6