Abstract
Purpose
Veliparib (V), an oral poly(ADP-ribose) polymerase (PARP) inhibitor, potentiates effects of alkylating agents and topoisomerase inhibitors in preclinical tumor models. We conducted a phase I trial of V with iv cyclophosphamide (C) and V plus iv doxorubicin (A) and C.
Methods
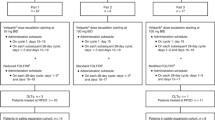
Objectives were to establish the maximum tolerated dose (MTD) of the combinations, characterize V pharmacokinetics (PK) in the presence and absence of C, measure PAR in peripheral blood mononuclear cells (PBMCs) and γH2AX in circulating tumor cells (CTCs). In Group 1, dose escalations of V from 10 to 50 mg every 12 h Days 1–4 plus C 450 to 750 mg/m2 Day 3 in 21-day cycles were evaluated. In Group 2, V doses ranged from 50 to 150 mg every 12 h Days 1–4 with AC (60/600 mg/m2) Day 3 in 21-day cycles. In Group 3, patients received AC Day 1 plus V Days 1–7, and in Group 4, AC Day 1 plus V Days 1–14 was given in 21-day cycles to evaluate effects on γH2AX foci.
Results
Eighty patients were enrolled. MTD was not reached for V and C. MTD for V and AC was V 100 mg every 12 h Days 1–4 with AC (60/600 mg/m2) Day 3 every 21 days. V PK appears to be dose-dependent and has no effect on the PK of C. Overall, neutropenia and anemia were the most common adverse events. Objective response in V and AC treated groups was 22% (11/49). Overall clinical benefit rate was 31% (25/80). PAR decreased in PBMCs. Percentage of γH2AX-positive CTCs increased after treatment with V and AC.
Conclusion
V and AC can be safely combined. Activity was observed in patients with metastatic breast cancer.


Similar content being viewed by others
References
Ame JC, Spenlehauer C, de Murcia G (2004) The PARP superfamily. BioEssays 26(8):882–893. https://doi.org/10.1002/bies.20085
Jagtap P, Szabo C (2005) Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov 4(5):421–440. https://doi.org/10.1038/nrd1718
Penning TD, Zhu GD, Gandhi VB, Gong J, Liu X, Shi Y, Klinghofer V, Johnson EF, Donawho CK, Frost DJ, Bontcheva-Diaz V, Bouska JJ, Osterling DJ, Olson AM, Marsh KC, Luo Y, Giranda VL (2009) Discovery of the Poly(ADP-ribose) polymerase (PARP) inhibitor 2-[(R)-2-methylpyrrolidin-2-yl]-1H-benzimidazole-4-carboxamide (ABT-888) for the treatment of cancer. J Med Chem 52(2):514–523. https://doi.org/10.1021/jm801171j
Donawho CK, Luo Y, Luo Y, Penning TD, Bauch JL, Bouska JJ, Bontcheva-Diaz VD, Cox BF, DeWeese TL, Dillehay LE, Ferguson DC, Ghoreishi-Haack NS, Grimm DR, Guan R, Han EK, Holley-Shanks RR, Hristov B, Idler KB, Jarvis K, Johnson EF, Kleinberg LR, Klinghofer V, Lasko LM, Liu X, Marsh KC, McGonigal TP, Meulbroek JA, Olson AM, Palma JP, Rodriguez LE, Shi Y, Stavropoulos JA, Tsurutani AC, Zhu GD, Rosenberg SH, Giranda VL, Frost DJ (2007) ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res 13(9):2728–2737. https://doi.org/10.1158/1078-0432.CCR-06-3039
Kummar S, Ji J, Morgan R, Lenz HJ, Puhalla SL, Belani CP, Gandara DR, Allen D, Kiesel B, Beumer JH, Newman EM, Rubinstein L, Chen A, Zhang Y, Wang L, Kinders RJ, Parchment RE, Tomaszewski JE, Doroshow JH (2012) A phase I study of veliparib in combination with metronomic cyclophosphamide in adults with refractory solid tumors and lymphomas. Clin Cancer Res 18(6):1726–1734. https://doi.org/10.1158/1078-0432.CCR-11-2821
LoRusso PM, Li J, Burger A, Heilbrun LK, Sausville EA, Boerner SA, Smith D, Pilat MJ, Zhang J, Tolaney SM, Cleary JM, Chen AP, Rubinstein L, Boerner JL, Bowditch A, Cai D, Bell T, Wolanski A, Marrero AM, Zhang Y, Ji J, Ferry-Galow K, Kinders RJ, Parchment RE, Shapiro GI (2016) Phase I safety, pharmacokinetic, and pharmacodynamic study of the poly(ADP-ribose) polymerase (PARP) inhibitor veliparib (ABT-888) in combination with irinotecan in patients with advanced solid tumors. Clin Cancer Res 22(13):3227–3237. https://doi.org/10.1158/1078-0432.CCR-15-0652
Atrafi F, Groen HJM, Byers LA, Garralda E, Lolkema MP, Sangha RS, Viteri S, Chae YK, Camidge DR, Gabrail NY, Hu B, Tian T, Nuthalapati S, Hoening E, He L, Komarnitsky P, Calles A (2019) A phase I dose-escalation study of veliparib combined with carboplatin and etoposide in patients with extensive-stage small cell lung cancer and other solid tumors. Clin Cancer Res 25(2):496–505. https://doi.org/10.1158/1078-0432.CCR-18-2014
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V, International Harmonization Project on Lymphoma (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25(5):579–586. https://doi.org/10.1200/JCO.2006.09.2403
Emmenegger U, Shaked Y, Man S, Bocci G, Spasojevic I, Francia G, Kouri A, Coke R, Cruz-Munoz W, Ludeman SM, Colvin OM, Kerbel RS (2007) Pharmacodynamic and pharmacokinetic study of chronic low-dose metronomic cyclophosphamide therapy in mice. Mol Cancer Ther 6(8):2280–2289. https://doi.org/10.1158/1535-7163.MCT-07-0181
Parise RA, Shawaqfeh M, Egorin MJ, Beumer JH (2008) Liquid chromatography-mass spectrometric assay for the quantitation in human plasma of ABT-888, an orally available, small molecule inhibitor of poly(ADP-ribose) polymerase. J Chromatogr B Analyt Technol Biomed Life Sci 872(1–2):141–147. https://doi.org/10.1016/j.jchromb.2008.07.032
Ji J, Kinders RJ, Zhang Y, Rubinstein L, Kummar S, Parchment RE, Tomaszewski JE, Doroshow JH (2011) Modeling pharmacodynamic response to the poly(ADP-Ribose) polymerase inhibitor ABT-888 in human peripheral blood mononuclear cells. PLoS ONE 6(10):e26152. https://doi.org/10.1371/journal.pone.0026152
Wang LH, Pfister TD, Parchment RE, Kummar S, Rubinstein L, Evrard YA, Gutierrez ME, Murgo AJ, Tomaszewski JE, Doroshow JH, Kinders RJ (2010) Monitoring drug-induced gammaH2AX as a pharmacodynamic biomarker in individual circulating tumor cells. Clin Cancer Res 16(3):1073–1084. https://doi.org/10.1158/1078-0432.CCR-09-2799
Li X, Delzer J, Voorman R, de Morais SM, Lao Y (2011) Disposition and drug-drug interaction potential of veliparib (ABT-888), a novel and potent inhibitor of poly(ADP-ribose) polymerase. Drug Metab Dispos 39(7):1161–1169. https://doi.org/10.1124/dmd.110.037820
Beijnen JH, Schellens JH (2004) Drug interactions in oncology. Lancet Oncol 5(8):489–496. https://doi.org/10.1016/S1470-2045(04)01528-1
Rodler ET, Kurland BF, Griffin M, Gralow JR, Porter P, Yeh RF, Gadi VK, Guenthoer J, Beumer JH, Korde L, Strychor S, Kiesel BF, Linden HM, Thompson JA, Swisher E, Chai X, Shepherd S, Giranda V, Specht JM (2016) Phase I study of veliparib (ABT-888) combined with cisplatin and vinorelbine in advanced triple-negative breast cancer and/or BRCA mutation-associated breast cancer. Clin Cancer Res 22(12):2855–2864. https://doi.org/10.1158/1078-0432.CCR-15-2137
Stoller R, Schmitz JC, Ding F, Puhalla S, Belani CP, Appleman L, Lin Y, Jiang Y, Almokadem S, Petro D, Holleran J, Kiesel BF, Ken Czambel R, Carneiro BA, Kontopodis E, Hershberger PA, Rachid M, Chen A, Chu E, Beumer JH (2017) Phase I study of veliparib in combination with gemcitabine. Cancer Chemother Pharmacol 80(3):631–643. https://doi.org/10.1007/s00280-017-3409-3
Niu J, Scheuerell C, Mehrotra S, Karan S, Puhalla S, Kiesel BF, Ji J, Chu E, Gopalakrishnan M, Ivaturi V, Gobburu J, Beumer JH (2017) Parent-metabolite pharmacokinetic modeling and pharmacodynamics of veliparib (ABT-888), a PARP inhibitor, in patients with BRCA 1/2-mutated cancer or PARP-sensitive tumor types. J Clin Pharmacol 57(8):977–987. https://doi.org/10.1002/jcph.892
Berlin J, Ramanathan RK, Strickler JH, Subramaniam DS, Marshall J, Kang YK, Hetman R, Dudley MW, Zeng J, Nickner C, Xiong H, Komarnitsky P, Shepherd SP, Hurwitz H, Lenz HJ (2018) A phase 1 dose-escalation study of veliparib with bimonthly FOLFIRI in patients with advanced solid tumours. Br J Cancer 118(7):938–946. https://doi.org/10.1038/s41416-018-0003-3
Kummar S, Oza AM, Fleming GF, Sullivan DM, Gandara DR, Naughton MJ, Villalona-Calero MA, Morgan RJ Jr, Szabo PM, Youn A, Chen AP, Ji J, Allen DE, Lih CJ, Mehaffey MG, Walsh WD, McGregor PM 3rd, Steinberg SM, Williams PM, Kinders RJ, Conley BA, Simon RM, Doroshow JH (2015) Randomized trial of oral cyclophosphamide and veliparib in high-grade serous ovarian, primary peritoneal, or fallopian tube cancers, or BRCA-mutant ovarian cancer. Clin Cancer Res 21(7):1574–1582. https://doi.org/10.1158/1078-0432.CCR-14-2565
Kummar S, Wade JL, Oza AM, Sullivan D, Chen AP, Gandara DR, Ji J, Kinders RJ, Wang L, Allen D, Coyne GO, Steinberg SM, Doroshow JH (2016) Randomized phase II trial of cyclophosphamide and the oral poly (ADP-ribose) polymerase inhibitor veliparib in patients with recurrent, advanced triple-negative breast cancer. Invest New Drugs 34(3):355–363. https://doi.org/10.1007/s10637-016-0335-x
Anampa J, Chen A, Wright J, Patel M, Pellegrino C, Fehn K, Sparano JA, Andreopoulou E (2018) Phase I trial of veliparib, a poly ADP ribose polymerase inhibitor, plus metronomic cyclophosphamide in metastatic HER2-negative breast cancer. Clin Breast Cancer 18(1):e135–e142. https://doi.org/10.1016/j.clbc.2017.08.013
Acknowledgements
We acknowledge Dr. Ulf Niemeyer, of Niomech -IIT GmbH, Bielefeld, Germany for the generous gift of PBOX-d4 (internal standard) for analysis of 4-OH-CPA.
Funding
Supported by Rutgers Cancer Institute of New Jersey Cancer Center Support Grant/Core Grant and NCI grants (U01-CA-132194-01, UM1-CA-186716, UM1-CA-186690, U01-CA099168, and R50 CA211241). This project used the UPCI Cancer Pharmacokinetics and Pharmacodynamics Facility and was supported in part by award P30-CA47904.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A. Tan has received research grants from Arvinas, Deciphera, Daiichi-Sankyo, Genentech, Merck, and Pfizer; advisory board member/paid consultant for Athenex, AstraZeneca, Eisai, G1 Therapeutics, Novartis, and Immunomedics. M. Stein has received research grants from Merck, Exelixis, Oncoceutics, Janssen, Medivation/Astellas, Advaxis, Suzhou Kintor, Harpoon, Bristol-Meyers Squibb, Genocea, Eli Lilly, Seattle Genetics and Xencor. R. Moss is an employee for Bristol-Myers Squibb. J. Malhotra has received commercial research grants from AstraZeneca, Beyond Spring, Bristol-Myers Squibb, Biohaven and Pfizer. J. Aisner is on the DMC for EMD Serono. J. Mehnert has received research grants from Merck, EMD Serono, Pfizer, Genentech, Amgen, Boehringer Ingelheim, Array BioPharma, Immunocore, AstraZeneca, Incyte, Macrogenics, Bristol-Myers Squibb, Novartis, and Polynoma. No potential conflicts of interest were disclosed by the other authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tan, A.R., Chan, N., Kiesel, B.F. et al. A phase I study of veliparib with cyclophosphamide and veliparib combined with doxorubicin and cyclophosphamide in advanced malignancies. Cancer Chemother Pharmacol 89, 49–58 (2022). https://doi.org/10.1007/s00280-021-04350-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-021-04350-x