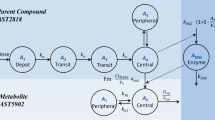
Abstract
Purpose
To examine the single- and multiple-dose pharmacokinetics (PK), CYP3A inhibition potential of ipatasertib, and effect of food on PK of ipatasertib in patients with refractory solid tumors and a dedicated food effect assessment in healthy subjects.
Methods
The Phase I dose-escalation study enrolled patients with solid tumors in a standard 3 + 3 design with a 1 week washout after the first dose, followed by once-daily dosing on a 3-week-on/1-week-off schedule. In the expansion cohort, the effect of ipatasertib on CYP3A substrate (midazolam) was assessed by examining the change in midazolam exposure when dosed in the absence and presence of steady-state ipatasertib at 600 mg. The effect of food on ipatasertib PK was studied with ipatasertib administered in fed or fasted state (6 patients from Phase I patient study and 18 healthy subjects from the dedicated food effect study).
Results
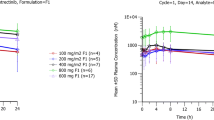
Ipatasertib was generally well tolerated at doses up to 600 mg given daily for 21 days. Ipatasertib showed rapid absorption (tmax, 0.5–3 h), was dose-proportional over a range of 200–800 mg, had a median half-life (range) of 45.0 h (27.8–66.9 h), and had approximately two-fold accumulation following once-daily dosing. Midazolam exposure (AUC0-∞) increased by 2.2-fold in the presence of ipatasertib. PK was comparable in subjects administered ipatasertib in a fed or fasted state.
Conclusion
Ipatasertib exhibited rapid absorption and was dose-proportional over a broad dose range. Ipatasertib appeared to be a moderate CYP3A inhibitor when administered at 600 mg and could be administered with or without food in clinical studies.
Trail registration
NCT01090960 (registered March 23, 2010); NCT02536391 (registered August 31, 2015).



Similar content being viewed by others
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Bellacosa A, de Feo D, Godwin AK, Bell DW, Cheng JQ, Altomare DA, Wan M, Dubeau L, Scambia G, Masciullo V, Ferrandina G, Benedetti Panici P, Mancuso S, Neri G, Testa JR (1995) Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int J cancer 64(4):280–285
Manning BD, Cantley LC (2007) AKT/PKB signaling: navigating downstream. Cell 129(7):1261–1274. https://doi.org/10.1016/j.cell.2007.06.009
Jiang BH, Liu LZ (2008) AKT signaling in regulating angiogenesis. Curr Cancer Drug Targets 8(1):19–26
Tokunaga E, Oki E, Egashira A, Sadanaga N, Morita M, Kakeji Y, Maehara Y (2008) Deregulation of the Akt pathway in human cancer. Curr Cancer Drug Targets 8(1):27–36. https://doi.org/10.2174/156800908783497140
Robey RB, Hay N (2009) Is Akt the “Warburg kinase”?-Akt-energy metabolism interactions and oncogenesis. Semin Cancer Biol 19(1):25–31. https://doi.org/10.1016/j.semcancer.2008.11.010
Hay N (2005) The Akt-mTOR tango and its relevance to cancer. Cancer Cell 8(3):179–183. https://doi.org/10.1016/j.ccr.2005.08.008
Cheng JQ, Lindsley CW, Cheng GZ, Yang H, Nicosia SV (2005) The Akt/PKB pathway: molecular target for cancer drug discovery. Oncogene 24(50):7482–7492. https://doi.org/10.1038/sj.onc.1209088
Yan Y, Serra V, Prudkin L, Scaltriti M, Murli S, Rodriguez O, Guzman M, Sampath D, Nannini M, Xiao Y, Wagle MC, Wu JQ, Wongchenko M, Hampton G, Ramakrishnan V, Lackner MR, Saura C, Roda D, Cervantes A, Tabernero J, Patel P, Baselga J (2013) Evaluation and clinical analyses of downstream targets of the Akt inhibitor GDC-0068. Clin Cancer Res 19(24):6976–6986. https://doi.org/10.1158/1078-0432.CCR-13-0978
Lin J, Sampath D, Nannini MA, Lee BB, Degtyarev M, Oeh J, Savage H, Guan Z, Hong R, Kassees R, Lee LB, Risom T, Gross S, Liederer BM, Koeppen H, Skelton NJ, Wallin JJ, Belvin M, Punnoose E, Friedman LS, Lin K (2013) Targeting activated Akt with GDC-0068, a novel selective Akt inhibitor that is efficacious in multiple tumor models. Clin Cancer Res 19(7):1760–1772. https://doi.org/10.1158/1078-0432.CCR-12-3072
Blake JF, Xu R, Bencsik JR, Xiao D, Kallan NC, Schlachter S, Mitchell IS, Spencer KL, Banka AL, Wallace EM, Gloor SL, Martinson M, Woessner RD, Vigers GP, Brandhuber BJ, Liang J, Safina BS, Li J, Zhang B, Chabot C, Do S, Lee L, Oeh J, Sampath D, Lee BB, Lin K, Liederer BM, Skelton NJ (2012) Discovery and preclinical pharmacology of a selective ATP-competitive Akt inhibitor (GDC-0068) for the treatment of human tumors. J Med Chem 55(18):8110–8127. https://doi.org/10.1021/jm301024w
de Bono JS, De Giorgi U, Rodrigues DN, Massard C, Bracarda S, Font A, ArranzArija JA, Shih KC, Radavoi GD, Xu N, Chan WY, Ma H, Gendreau S, Riisnaes R, Patel PH, Maslyar DJ, Jinga V (2019) Randomized phase II study evaluating Akt blockade with ipatasertib, in combination with abiraterone, in patients with metastatic prostate cancer with and without PTEN loss. Clin Cancer Res 25(3):928–936. https://doi.org/10.1158/1078-0432.CCR-18-0981
Kim SB, Dent R, Im SA, Espie M, Blau S, Tan AR, Isakoff SJ, Oliveira M, Saura C, Wongchenko MJ, Kapp AV, Chan WY, Singel SM, Maslyar DJ, Baselga J, investigators L, (2017) Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 18(10):1360–1372. https://doi.org/10.1016/S1470-2045(17)30450-3
FDA (2002) Guidance for industry. Food-effect bioavailability and fed bioequivalence studies. https://www.fda.gov/media/70945/download.
Saura C, Roda D, Rosello S, Oliveira M, Macarulla T, Perez-Fidalgo JA, Morales-Barrera R, Sanchis-Garcia JM, Musib L, Budha N, Zhu J, Nannini M, Chan WY, SanabriaBohorquez SM, Meng RD, Lin K, Yan Y, Patel P, Baselga J, Tabernero J, Cervantes A (2017) A first-in-human phase I study of the ATP-competitive AKT inhibitor ipatasertib demonstrates robust and safe targeting of AKT in patients with solid tumors. Cancer Discov 7(1):102–113. https://doi.org/10.1158/2159-8290.CD-16-0512
Wu CY, Benet LZ (2005) Predicting drug disposition via application of BCS: transport/absorption/ elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res 22(1):11–23
Fleisher D, Li C, Zhou Y, Pao LH, Karim A (1999) Drug, meal and formulation interactions influencing drug absorption after oral administration. Clin Implic Clin pharmacokinet 36(3):233–254. https://doi.org/10.2165/00003088-199936030-00004
Acknowledgements
The study was funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland, and Genentech, Inc., a member of the Roche Group, South San Francisco, CA, USA. The authors would also like to thank all participating patients and their families. Support for third-party writing assistance for this manuscript was provided by F. Hoffmann-La Roche Ltd.
Funding
The study was funded by F. Hoffmann-La Roche Ltd., Basel, Switzerland, and Genentech, Inc., a member of the Roche Group, South San Francisco, CA, USA.
Author information
Authors and Affiliations
Contributions
VM contributed to data analysis and interpretation, writing the initial draft, revision of the manuscript for critically important content, and approval of final manuscript submission. NB, RS, JH contributed to data analysis and interpretation, revision of the manuscript for critically important content, and approval of final manuscript submission. BL contributed to study design, revision of the manuscript for critically important content, and approval of final manuscript submission. RM, PP contributed to study design, data analysis and interpretation, revision of the manuscript for critically important content, and approval of final manuscript submission. YD contributed to study design, data collection, revision of the manuscript for critically important content, and approval of final manuscript submission. AC, JT contributed to the study design, data collection revision of the manuscript for critically important content and approval of final manuscript submission. LM contributed to study design, data analysis and interpretation, writing the initial draft, revision of the manuscript for critically important content, and approval of final manuscript submission.
Corresponding author
Ethics declarations
Conflict of interest
Authors Vikram Malhi, Nageshwar Budha, Rucha Sane, Jian Huang, Bianca Liederer, Raymond Meng, Premal Patel, Yuzhong Deng, Luna Musib are or were Genentech employees and owned Roche stock at the time of this study. Author Josep Tabernero reports personal financial interest in form of scientific consultancy role for Array Biopharma, AstraZeneca, Bayer, Boehringer Ingelheim, Chugai, Daiichi Sankyo, F. Hoffmann-La Roche Ltd, Genentech, Inc, HalioDX SAS, Hutchison MediPharma International, Ikena Oncology, IQVIA, Lilly, Menarini, Merck Serono, Merus, MSD, Mirati, Neophore, Novartis, Orion Biotechnology, Peptomyc, Pfizer, Pierre Fabre, Samsung Bioepis, Sanofi, Seattle Genetics, Servier, Taiho, Tessa Therapeutics and TheraMyc. Author Andres Cervantes reports institutional research funding from Astellas, Bayer, BeiGene, Bristol-Myers Squibb, Fibrogen, Genentech, Lilly, Merck Serono, Merck Sharp & Dohme, Novartis, Roche, Servier, Takeda; advisory board or speaker fees: Astellas, Merck Serono, Roche, Servier, Takeda.
Ethical approval
Studies were conducted in accordance with good clinical practice guidelines, applicable laws, and regulations, and in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Regulatory authorities and institutional review boards approved the study. Additional approval for the Phase I patient study was obtained from CEIC Hospital Vall D’Hebron, PASSEIG DE LA VALL D'HEBRON S/N 119-129, 08035, Barcelona, Spain and EC/IRB Approval Date 08-Jan-2010, Hospital Clínico Universitario de Valencia; CEIC, Pabellón B. Piso 1 Avda. Vicente Blasco ibáñez, 17, 46010, Valencia, VALENCIA, SPAIN. EC/IRB Approval Date 08-Jan-2010. The Phase 1 healthy subject study was approved by Schulman Associates Institutional Review Board, Address: 4445 Lake Forest Drive, Suite 300 Cincinnati, Ohio 45242. This article does not contain any studies with animals performed by any of the authors.
Informed consent
All subjects in the studies provided written informed consent prior to enrollment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Malhi, V., Budha, N., Sane, R. et al. Single- and multiple-dose pharmacokinetics, potential for CYP3A inhibition, and food effect in patients with cancer and healthy subjects receiving ipatasertib. Cancer Chemother Pharmacol 88, 921–930 (2021). https://doi.org/10.1007/s00280-021-04344-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-021-04344-9