Abstract
Purpose
CHMFL-KIT-110, a selective c-KIT kinase inhibitor for gastrointestinal stromal tumors (GISTs), possesses a poorly water-soluble, limiting the further development of the drug. This study was to investigate the antitumor efficacy of CHMFL-KIT-110 and CHMFL-KIT-110 solid dispersion (laboratory code: HYGT-110 SD) in GIST tumor xenograft models and to explore the PK/PD relationship of HYGT-110 SD.
Methods
Plasma concentrations of HYGT-110 and HYGT-110 SD were determined by LC–MS/MS in KM mice. Antitumor activity was evaluated by measuring tumor volume and weight in c-KIT-dependent GIST xenograft models. PK/PD relationship was assessed by LC–MS/MS and Western Blot in the GIST-T1 xenografted mice.
Results
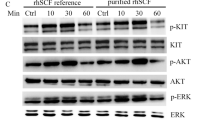
HYGT-110 exhibited a low oral bioavailability (10.91%) in KM mice. Compared with HYGT-110 treatment, the Cmax and AUC0-t of HYGT-110 SD in mice plasma were substantially increased by 18.81 and 6.76-fold, respectively. HYGT-110 SD (10, 30, and 100 mg/kg/day) also could dose-dependently decrease the tumor volume and weight in the GIST-882 cell-inoculated xenograft mouse models and show 86.35% tumor growth inhibition (TGI) at 28 days at a 25 mg/kg bid dosage in the GIST-T1 cell-inoculated xenograft mouse model. The free concentration of HYGT-110 in plasma was closely correlated with the inhibition of c-KIT phosphorylation levels in tumor tissues.
Conclusions
In comparison with the HPMC formulation, both improved PK and PD characteristics of the solid dispersion formulation of CHMFL-KIT-110 were observed in in vivo animal experiments.






Similar content being viewed by others
References
Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y (1998) Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279(5350):577–580. https://doi.org/10.1126/science.279.5350.577
Von Mehren M, Joensuu H (2018) Gastrointestinal stromal tumors. J Clin Oncol 36(2):136–143. https://doi.org/10.1200/JCO.2017.74.9705
Cassier PA, Dufresne A, Arifi S, El Sayadi H, Ray-Coquard I, Bringuier PP, Scoazec JY, Alberti L, Blay JY (2008) Novel approaches to gastrointestinal stromal tumors resistant to imatinib and sunitinib. Curr Gastroenterol Rep 10(6):555–561. https://doi.org/10.1007/s11894-008-0102-z
Martin-Broto J, Moura DS (2020) New drugs in gastrointestinal stromal tumors. Curr Opin Oncol 32(4):314–320. https://doi.org/10.1097/CCO.0000000000000642
George S, Wang Q, Heinrich MC, Corless CL, Zhu M, Butrynski JE, Morgan JA, Wagner AJ, Choy E, Tap WD, Yap JT, Van den Abbeele AD, Manola JB, Solomon SM, Fletcher JA, von Mehren M, Demetri GD (2012) Efficacy and safety of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of imatinib and sunitinib: a multicenter phase II trial. J Clin Oncol 30(19):2401–2407. https://doi.org/10.1200/JCO.2011.39.9394
Gupta R, Maitland ML (2011) Sunitinib, hypertension, and heart failure: a model for kinase inhibitor-mediated cardiotoxicity. Curr Hypertens Rep 13(6):430–435. https://doi.org/10.1007/s11906-011-0229-4
Rutkowski P, Stepniak J (2016) The safety of regorafenib for the treatment of gastrointestinal stromal tumors. Expert Opin Drug Saf 15(1):105–116. https://doi.org/10.1517/14740338.2016.1122754
Wang Q, Liu F, Wang B, Zou F, Chen C, Liu X, Wang A, Qi S, Wang W, Qi Z, Zhao Z, Hu Z, Wang W, Wang L, Zhang S, Wang Y, Liu J, Liu Q (2016) Discovery of N-(3-((1-Isonicotinoylpiperidin-4-yl)oxy)-4-methylphenyl)-3-(trifluoromethyl)benz amide (CHMFL-KIT-110) as a selective, potent, and orally available type II c-KIT kinase inhibitor for gastrointestinal stromal tumors (GISTs). J Med Chem 59(8):3964–3979. https://doi.org/10.1021/acs.jmedchem.6b00200
Huang Y, Dai WG (2014) Fundamental aspects of solid dispersion technology for poorly soluble drugs. Acta Pharm Sin B 4(1):18–25. https://doi.org/10.1016/j.apsb.2013.11.001
Schittny A, Philipp-Bauer S, Detampel P, Huwyler J, Puchkov M (2020) Mechanistic insights into effect of surfactants on oral bioavailability of amorphous solid dispersions. J Control Release 320:214–225. https://doi.org/10.1016/j.jconrel.2020.01.031
Wu Y, Chen D, Wang X, Sun H, Huo M (2020) Preparation, physicochemical properties and pharmacokinetics in rats of CHMFL-KIT-110 solid dispersions. J China Pharm Univ 51(06):688–695 ((In Chinese))
Derendorf H, Lesko LJ, Chaikin P, Colburn WA, Lee P, Miller R, Powell R, Rhodes G, Stanski D, Venitz J (2000) Pharmacokinetic/pharmacodynamic modeling in drug research and development. J Clin Pharmacol 40(12 Pt 2):1399–1418
Tuntland T, Ethell B, Kosaka T, Blasco F, Zang RX, Jain M, Gould T, Hoffmaster K (2014) Implementation of pharmacokinetic and pharmacodynamic strategies in early research phases of drug discovery and development at Novartis Institute of Biomedical Research. Front Pharmacol 5:174. https://doi.org/10.3389/fphar.2014.00174
Pilla Reddy V, Anjum R, Grondine M, Smith A, Bhavsar D, Barry E, Guichard SM, Shao W, Kettle JG, Brown C, Banks E, Jones RDO (2020) The pharmacokinetic-pharmacodynamic (PKPD) relationships of AZD3229, a Novel and selective inhibitor of KIT, in a range of mouse xenograft models of GIST. Clin Cancer Res 26(14):3751–3759. https://doi.org/10.1158/1078-0432.CCR-19-2848
Floris G, Debiec-Rychter M, Wozniak A, Stefan C, Normant E, Faa G, Machiels K, Vanleeuw U, Sciot R, Schoffski P (2011) The heat shock protein 90 inhibitor IPI-504 induces KIT degradation, tumor shrinkage, and cell proliferation arrest in xenograft models of gastrointestinal stromal tumors. Mol Cancer Ther 10(10):1897–1908. https://doi.org/10.1158/1535-7163.MCT-11-0148
Nagano T, Yasunaga M, Goto K, Kenmotsu H, Koga Y, Kuroda J, Nishimura Y, Sugino T, Nishiwaki Y, Matsumura Y (2009) Antitumor activity of NK012 combined with cisplatin against small cell lung cancer and intestinal mucosal changes in tumor-bearing mouse after treatment. Clin Cancer Res 15(13):4348–4355. https://doi.org/10.1158/1078-0432.CCR-08-3334
Qin J, Yuan J, Li L, Liu H, Qin R, Qin W, Chen B, Wang H, Wu K (2009) In vitro and in vivo inhibitory effect evaluation of cyclooxygenase-2 inhibitors, antisense cyclooxygenase-2 cDNA, and their combination on the growth of human bladder cancer cells. Biomed Pharmacother 63(3):241–248. https://doi.org/10.1016/j.biopha.2008.04.007
Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, Schreck RE, Abrams TJ, Ngai TJ, Lee LB, Murray LJ, Carver J, Chan E, Moss KG, Haznedar JO, Sukbuntherng J, Blake RA, Sun L, Tang C, Miller T, Shirazian S, McMahon G, Cherrington JM (2003) In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res 9(1):327–337
Wu Y, Wang B, Wang J, Qi S, Zou F, Qi Z, Liu F, Liu Q, Chen C, Hu C, Hu Z, Wang A, Wang L, Wang W, Ren T, Cai Y, Bai M, Liu Q, Liu J (2019) Discovery of 2-(4-Chloro-3-(trifluoromethyl)phenyl)-N-(4-((6,7-dimethoxyquinolin-4-yl)oxy)phen yl)acetamide (CHMFL-KIT-64) as a novel orally available potent inhibitor against broad-spectrum mutants of c-KIT kinase for gastrointestinal stromal tumors. J Med Chem 62(13):6083–6101. https://doi.org/10.1021/acs.jmedchem.9b00280
Ferreira NH, Ribeiro AB, Rinaldi-Neto F, Fernandes FS, do Nascimento S, Braz WR, Nassar EJ, Tavares DC, (2020) Anti-melanoma activity of indomethacin incorporated into mesoporous silica nanoparticles. Pharm Res 37(9):172. https://doi.org/10.1007/s11095-020-02903-y
Trainor GL (2007) The importance of plasma protein binding in drug discovery. Expert Opin Drug Discov 2(1):51–64. https://doi.org/10.1517/17460441.2.1.51
Guan J, Jin L, Liu Q, Xu H, Wu H, Zhang X, Mao S (2019) Exploration of supersaturable lacidipine ternary amorphous solid dispersion for enhanced dissolution and in vivo absorption. Eur J Pharm Sci 139:105043. https://doi.org/10.1016/j.ejps.2019.105043
Cowley S, Paterson H, Kemp P, Marshall CJ (1994) Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell 77(6):841–852. https://doi.org/10.1016/0092-8674(94)90133-3
Daouti S, Wang H, Li WH, Higgins B, Kolinsky K, Packman K, Specian A Jr, Kong N, Huby N, Wen Y, Xiang Q, Podlaski FJ, He Y, Fotouhi N, Heimbrook D, Niu H (2009) Characterization of a novel mitogen-activated protein kinase kinase 1/2 inhibitor with a unique mechanism of action for cancer therapy. Cancer Res 69(5):1924–1932. https://doi.org/10.1158/0008-5472.CAN-08-2627
Liu L, Chen J, Cao M, Wang J, Wang S (2019) NO donor inhibits proliferation and induces apoptosis by targeting PI3K/AKT/mTOR and MEK/ERK pathways in hepatocellular carcinoma cells. Cancer Chemother Pharmacol 84(6):1303–1314. https://doi.org/10.1007/s00280-019-03965-5
Saini KS, Loi S, de Azambuja E, Metzger-Filho O, Saini ML, Ignatiadis M, Dancey JE, Piccart-Gebhart MJ (2013) Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK pathways in the treatment of breast cancer. Cancer Treat Rev 39(8):935–946. https://doi.org/10.1016/j.ctrv.2013.03.009
Bohnert T, Gan LS (2013) Plasma protein binding: from discovery to development. J Pharm Sci 102(9):2953–2994. https://doi.org/10.1002/jps.23614
Acknowledgements
These authors acknowledge staff members' help from the Hefei Blooming Drug Safety Evaluation Co., Ltd, and Institute of Clinical Pharmacology, Anhui Medical University, in conducting these studies. The authors would like to acknowledge Hongzhang Sun for performing the formulation study and manufacturing work of the test article. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, S., Wang, C., Wang, X. et al. Antitumor efficacy of CHMFL-KIT-110 solid dispersion in mouse xenograft models of human gastrointestinal stromal tumors. Cancer Chemother Pharmacol 88, 795–804 (2021). https://doi.org/10.1007/s00280-021-04332-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-021-04332-z