Abstract
Purpose
The combination of docetaxel, cisplatin and 5-fluorouracil (DCF) is a newly developed chemotherapy regimen for esophageal cancer. Severe neutropenia is dose-limiting toxicity of docetaxel and it is well known to be frequently occurred during DCF chemotherapy. This study aimed to investigate the relationship between severe neutropenia and genetic polymorphisms in patients treated with preoperative DCF chemotherapy.
Methods
A total of 158 patients were investigated for their absolute neutrophil count (ANC) within the first cycle of DCF chemotherapy at the National Cancer Center (NCC) Hospital East. DNA samples obtained from the NCC Biobank Registry were used for the analysis of nine genetic polymorphisms related to docetaxel pharmacokinetics. These genotypes were evaluated for their association with severe neutropenia, and further their risk factors were examined using a multivariate logistic regression.
Results
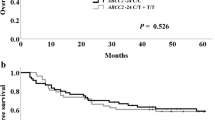
A total 81 (51.3%) patients developed severe neutropenia. Multivariate analysis revealed that age (OR 1.054; CI 1.008–1.102, P = 0.022), baseline ANC (OR 1.019; CI 1.002–1.037, P = 0.030), ABCB1 3435C>T (OR 2.191; CI 1.087–4.417, P = 0.028) and ABCC2 *+9383C>G (OR 2.342; CI 1.108–4.948, P = 0.026) were significant risk factors for severe neutropenia development. The results from this study showed that age, ANC, ABCB1 3435C>T, and ABCC2 *+9383 G>C increased the incidence of severe neutropenia with the number of identified risk factors.
Conclusions
In addition to age and baseline ANC, ABCB1 3435C>T and ABCC2 *+9383C>G were identified as independent predictors for severe neutropenia in esophageal cancer patients treated with DCF chemotherapy.
Similar content being viewed by others
References
Hara H, Tahara M, Daiko H, Kato K, Igaki H, Kadowaki S, Tanaka Y, Hamamoto Y, Matsushita H, Nagase M, Hosoya Y (2013) Phase II feasibility study of preoperative chemotherapy with docetaxel, cisplatin, and fluorouracil for esophageal squamous cell carcinoma. Cancer Sci 104(11):1455–1460. https://doi.org/10.1111/cas.12274
Aslani A, Smith RC, Allen BJ, Pavlakis N, Levi JA (2000) The predictive value of body protein for chemotherapy-induced toxicity. Cancer 88(4):796–803. https://doi.org/10.1002/(sici)1097-0142(20000215)88:4%3c796:aid-cncr10%3e3.0.co;2-p
Voog E, Bienvenu J, Warzocha K, Moullet I, Dumontet C, Thieblemont C, Monneret G, Gutowski MC, Coiffier B, Salles G (2000) Factors that predict chemotherapy-induced myelosuppression in lymphoma patients: role of the tumor necrosis factor ligand-receptor system. J Clin Oncol 18(2):325–331. https://doi.org/10.1200/JCO.2000.18.2.325
Intragumtornchai T, Sutheesophon J, Sutcharitchan P, Swasdikul D (2000) A predictive model for life-threatening neutropenia and febrile neutropenia after the first course of CHOP chemotherapy in patients with aggressive non-Hodgkin’s lymphoma. Leuk Lymphoma 37(3–4):351–360. https://doi.org/10.3109/10428190009089435
Lyman GH, Kuderer NM, Crawford J, Wolff DA, Culakova E, Poniewierski MS, Dale DC (2011) Predicting individual risk of neutropenic complications in patients receiving cancer chemotherapy. Cancer 117(9):1917–1927. https://doi.org/10.1002/cncr.25691
Schwenkglenks M, Pettengell R, Jackisch C, Paridaens R, Constenla M, Bosly A, Szucs TD, Leonard R (2011) Risk factors for chemotherapy-induced neutropenia occurrence in breast cancer patients: data from the INC-EU Prospective Observational European Neutropenia Study. Support Care Cancer 19(4):483–490. https://doi.org/10.1007/s00520-010-0840-y
Nomura H, Hatogai K, Maki Y, Mochizuki N, Tanaka M, Saito S, Daiko H, Kojima T, Kawasaki T (2019) Risk factors for febrile neutropenia in neoadjuvant docetaxel, cisplatin, and 5-fluorouracil chemotherapy for esophageal cancer. Support Care Cancer. https://doi.org/10.1007/s00520-019-05001-x
Donkor KN, Selim JH, Waworuntu A, Lewis K (2017) Safety and efficacy of pegfilgrastim when given less than 14 days before the next chemotherapy cycle: review of every 14-day chemotherapy regimen containing 5-FU continuous infusion. Ann Pharmacother 51(10):840–847. https://doi.org/10.1177/1060028017714554
Yoshida Y, Komori K, Aoki M, Sandou M, Takagi M, Uejima E (2018) Efficacy of pegfilgrastim administration in patients with esophageal cancer treated with docetaxel, cisplatin, and 5-fluorouracil. Pharmazie 73(10):613–616. https://doi.org/10.1691/ph.2018.8576
Nakamura K, Kato K, Igaki H, Ito Y, Mizusawa J, Ando N, Udagawa H, Tsubosa Y, Daiko H, Hironaka S, Fukuda H, Kitagawa Y, Japan Esophageal Oncology Group/Japan Clinical Oncology G (2013) Three-arm phase III trial comparing cisplatin plus 5-FU (CF) versus docetaxel, cisplatin plus 5-FU (DCF) versus radiotherapy with CF (CF-RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT study). Jpn J Clin Oncol 43(7):752–755. https://doi.org/10.1093/jjco/hyt061
Ando N, Iizuka T, Ide H, Ishida K, Shinoda M, Nishimaki T, Takiyama W, Watanabe H, Isono K, Aoyama N, Makuuchi H, Tanaka O, Yamana H, Ikeuchi S, Kabuto T, Nagai K, Shimada Y, Kinjo Y, Fukuda H, Japan Clinical Oncology G (2003) Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study—JCOG9204. J Clin Oncol 21(24):4592–4596. https://doi.org/10.1200/JCO.2003.12.095
Ui T, Fujii H, Hosoya Y, Nagase M, Mieno MN, Mori M, Zuiki T, Saito S, Kurashina K, Haruta H, Matsumoto S, Niki T, Lefor A, Yasuda Y (2015) Comparison of preoperative chemotherapy using docetaxel, cisplatin and fluorouracil with cisplatin and fluorouracil in patients with advanced carcinoma of the thoracic esophagus. Dis Esophagus 28(2):180–187. https://doi.org/10.1111/dote.12187
Lorenzen S, Schuster T, Porschen R, Al-Batran SE, Hofheinz R, Thuss-Patience P, Moehler M, Grabowski P, Arnold D, Greten T, Muller L, Rothling N, Peschel C, Langer R, Lordick F (2009) Cetuximab plus cisplatin-5-fluorouracil versus cisplatin-5-fluorouracil alone in first-line metastatic squamous cell carcinoma of the esophagus: a randomized phase II study of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol 20(10):1667–1673. https://doi.org/10.1093/annonc/mdp069
Alberti P, Cavaletti G (2014) Management of side effects in the personalized medicine era: chemotherapy-induced peripheral neuropathy. Methods Mol Biol 1175:301–322. https://doi.org/10.1007/978-1-4939-0956-8_12
Sugishita M, Imai T, Kikumori T, Mitsuma A, Shimokata T, Shibata T, Morita S, Inada-Inoue M, Sawaki M, Hasegawa Y, Ando Y (2016) Pharmacogenetic association between GSTP1 genetic polymorphism and febrile neutropenia in Japanese patients with early breast cancer. Breast Cancer 23(2):195–201. https://doi.org/10.1007/s12282-014-0547-x
Tsuji D, Ikeda M, Yamamoto K, Nakamori H, Kim YI, Kawasaki Y, Otake A, Yokoi M, Inoue K, Hirai K, Nakamichi H, Tokou U, Shiokawa M, Itoh K (2016) Drug-related genetic polymorphisms affecting severe chemotherapy-induced neutropenia in breast cancer patients: a hospital-based observational study. Medicine 95(44):e5151. https://doi.org/10.1097/MD.0000000000005151
Evans WE, McLeod HL (2003) Pharmacogenomics–drug disposition, drug targets, and side effects. N Engl J Med 348(6):538–549. https://doi.org/10.1056/NEJMra020526
Watanabe A, Yamamoto K, Ioroi T, Hirata S, Harada K, Miyake H, Fujisawa M, Nakagawa T, Yano I, Hirai M (2017) Association of single nucleotide polymorphisms in STAT3, ABCB1, and ABCG2 with stomatitis in patients with metastatic renal cell carcinoma treated with sunitinib: a retrospective analysis in Japanese patients. Biol Pharm Bull 40(4):458–464. https://doi.org/10.1248/bpb.b16-00875
Oliva D, Nilsson M, Andersson BA, Sharp L, Lewin F, Laytragoon-Lewin N (2017) Single nucleotide polymorphisms might influence chemotherapy induced nausea in women with breast cancer. Clin Transl Radiat Oncol 2:1–6. https://doi.org/10.1016/j.ctro.2016.12.001
Jordheim LP, Ribrag V, Ghesquieres H, Pallardy S, Delarue R, Tilly H, Haioun C, Jardin F, Demangel D, Salles GA, Dumontet C (2015) Single nucleotide polymorphisms in ABCB1 and CBR1 can predict toxicity to R-CHOP type regimens in patients with diffuse non-Hodgkin lymphoma. Haematologica 100(5):e204–e206. https://doi.org/10.3324/haematol.2014.120113
de Weger VA, Beijnen JH, Schellens JH (2014) Cellular and clinical pharmacology of the taxanes docetaxel and paclitaxel—a review. Anticancer Drugs 25(5):488–494. https://doi.org/10.1097/CAD.0000000000000093
Choi JR, Kim JO, Kang DR, Shin JY, Zhang XH, Oh JE, Park JY, Kim KA, Kang JH (2015) Genetic variations of drug transporters can influence on drug response in patients treated with docetaxel chemotherapy. Cancer Res Treat 47(3):509–517. https://doi.org/10.4143/crt.2014.012
Tsai SM, Lin CY, Wu SH, Hou LA, Ma H, Tsai LY, Hou MF (2009) Side effects after docetaxel treatment in Taiwanese breast cancer patients with CYP3A4, CYP3A5, and ABCB1 gene polymorphisms. Clin Chim Acta Int J Clin Chem 404(2):160–165. https://doi.org/10.1016/j.cca.2009.03.038
Kim KP, Ahn JH, Kim SB, Jung KH, Yoon DH, Lee JS, Ahn SH (2012) Prospective evaluation of the drug-metabolizing enzyme polymorphisms and toxicity profile of docetaxel in Korean patients with operable lymph node-positive breast cancer receiving adjuvant chemotherapy. Cancer Chemother Pharmacol 69(5):1221–1227. https://doi.org/10.1007/s00280-011-1816-4
Leslie EM, Deeley RG, Cole SP (2005) Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol 204(3):216–237. https://doi.org/10.1016/j.taap.2004.10.012
Muto M, Onogawa T, Suzuki T, Ishida T, Rikiyama T, Katayose Y, Ohuchi N, Sasano H, Abe T, Unno M (2007) Human liver-specific organic anion transporter-2 is a potent prognostic factor for human breast carcinoma. Cancer Sci 98(10):1570–1576. https://doi.org/10.1111/j.1349-7006.2007.00570.x
Strange RC, Fryer AA (1999) The glutathione S-transferases: influence of polymorphism on cancer susceptibility. IARC Sci Publ 148:231–249
Kim HJ, Im SA, Keam B, Ham HS, Lee KH, Kim TY, Kim YJ, Oh DY, Kim JH, Han W, Jang IJ, Kim TY, Park IA, Noh DY (2015) ABCB1 polymorphism as prognostic factor in breast cancer patients treated with docetaxel and doxorubicin neoadjuvant chemotherapy. Cancer Sci 106(1):86–93. https://doi.org/10.1111/cas.12560
Kiyotani K, Mushiroda T, Kubo M, Zembutsu H, Sugiyama Y, Nakamura Y (2008) Association of genetic polymorphisms in SLCO1B3 and ABCC2 with docetaxel-induced leukopenia. Cancer Sci 99(5):967–972. https://doi.org/10.1111/j.1349-7006.2008.00765.x
Awada Z, Haider S, Tfayli A, Bazarbachi A, El-Saghir NS, Salem Z, Shamseddine A, Taher A, Zgheib NK (2013) Pharmacogenomics variation in drug metabolizing enzymes and transporters in relation to docetaxel toxicity in Lebanese breast cancer patients: paving the way for OMICs in low and middle income countries. OMICS 17(7):353–367. https://doi.org/10.1089/omi.2013.0019
Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, Lyman GH, Pettengell R, Tjan-Heijnen VC, Walewski J, Weber DC, Zielinski C, European Organisation for R, Treatment of C (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 47(1):8–32. https://doi.org/10.1016/j.ejca.2010.10.013
Smith TJ, Bohlke K, Lyman GH, Carson KR, Crawford J, Cross SJ, Goldberg JM, Khatcheressian JL, Leighl NB, Perkins CL, Somlo G, Wade JL, Wozniak AJ, Armitage JO, American Society of Clinical O (2015) Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 33(28):3199–3212. https://doi.org/10.1200/JCO.2015.62.3488
Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmoller J, Johne A, Cascorbi I, Gerloff T, Roots I, Eichelbaum M, Brinkmann U (2000) Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA 97(7):3473–3478. https://doi.org/10.1073/pnas.050585397
Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, Gottesman MM (2007) A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science 315(5811):525–528. https://doi.org/10.1126/science.1135308
Tran A, Jullien V, Alexandre J, Rey E, Rabillon F, Girre V, Dieras V, Pons G, Goldwasser F, Treluyer JM (2006) Pharmacokinetics and toxicity of docetaxel: role of CYP3A, MDR1, and GST polymorphisms. Clin Pharmacol Ther 79(6):570–580. https://doi.org/10.1016/j.clpt.2006.02.003
Chang H, Rha SY, Jeung HC, Im CK, Noh SH, Kim JJ, Chung HC (2010) Association of the ABCB1 3435C>T polymorphism and treatment outcomes in advanced gastric cancer patients treated with paclitaxel-based chemotherapy. Oncol Rep 23(1):271–278
Facchini L, Martino R, Ferrari A, Pinana JL, Valcarcel D, Barba P, Granell M, Delgado J, Briones J, Sureda A, Brunet S, Sierra J (2012) Degree of mucositis and duration of neutropenia are the major risk factors for early post-transplant febrile neutropenia and severe bacterial infections after reduced-intensity conditioning. Eur J Haematol 88(1):46–51. https://doi.org/10.1111/j.1600-0609.2011.01724.x
Lyman GH, Delgado DJ (2003) Risk and timing of hospitalization for febrile neutropenia in patients receiving CHOP, CHOP-R, or CNOP chemotherapy for intermediate-grade non-Hodgkin lymphoma. Cancer 98(11):2402–2409. https://doi.org/10.1002/cncr.11827
Lyman GH, Lyman CH, Agboola O (2005) Risk models for predicting chemotherapy-induced neutropenia. Oncologist 10(6):427–437. https://doi.org/10.1634/theoncologist.10-6-427
Dranitsaris G, Rayson D, Vincent M, Chang J, Gelmon K, Sandor D, Reardon G (2008) Identifying patients at high risk for neutropenic complications during chemotherapy for metastatic breast cancer with doxorubicin or pegylated liposomal doxorubicin: the development of a prediction model. Am J Clin Oncol 31(4):369–374. https://doi.org/10.1097/COC.0b013e318165c01d
Pettengell R, Bosly A, Szucs TD, Jackisch C, Leonard R, Paridaens R, Constenla M, Schwenkglenks M, Impact of Neutropenia in Chemotherapy-European Study G (2009) Multivariate analysis of febrile neutropenia occurrence in patients with non-Hodgkin lymphoma: data from the INC-EU Prospective Observational European Neutropenia Study. Br J Haematol 144(5):677–685. https://doi.org/10.1111/j.1365-2141.2008.07514.x
Hirasawa Y, Nakashima J, Sugihara T, Takizawa I, Gondo T, Nakagami Y, Horiguchi Y, Ohno Y, Namiki K, Ohori M, Tachibana M (2017) Development of a nomogram for predicting severe neutropenia associated with docetaxel-based chemotherapy in patients with castration-resistant prostate cancer. Clin Genitourin Cancer 15(1):176–181. https://doi.org/10.1016/j.clgc.2016.05.012
Yano R, Konno A, Watanabe K, Tsukamoto H, Kayano Y, Ohnaka H, Goto N, Nakamura T, Masada M (2013) Pharmacoethnicity of docetaxel-induced severe neutropenia: integrated analysis of published phase II and III trials. Int J Clin Oncol 18(1):96–104. https://doi.org/10.1007/s10147-011-0349-5
van der Wouden CH, van Rhenen MH, Jama WOM, Ingelman-Sundberg M, Lauschke VM, Konta L, Schwab M, Swen JJ, Guchelaar HJ (2019) Development of the PGx-passport: a panel of actionable germline genetic variants for pre-emptive pharmacogenetic testing. Clin Pharmacol Ther 106(4):866–873. https://doi.org/10.1002/cpt.1489
Funding
This work was supported by the University of Shizuoka academic research grant.
Author information
Authors and Affiliations
Contributions
Conception and design were performed by HN, DT, TaKo, TY, HD and SF. Acquisition of data was carried out by HN, KD, DT, HD, and TaKo. The analysis of SNPs was DT, HM and KI. Statistical analysis and interpretation of the data were carried out by NM. Drafting of the article was carried out by NH, DT, TaKo, ToKa, and SF. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval
The study protocol was approved by the institutional review board of the NCC, Japan in March 2017 (2016-384). These patients were consented to be used the clinical information and DNA sample in the National Cancer Center (NCC) Biobank Registry. The study design is displayed on the website for the National Cancer Center Hospital East, providing the relatives of deceased patients the opportunity to decline participation in the current study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nomura, H., Tsuji, D., Demachi, K. et al. ABCB1 and ABCC2 genetic polymorphism as risk factors for neutropenia in esophageal cancer patients treated with docetaxel, cisplatin, and 5-fluorouracil chemotherapy. Cancer Chemother Pharmacol 86, 315–324 (2020). https://doi.org/10.1007/s00280-020-04118-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-020-04118-9