Abstract
Purpose
This study was designed to assess the tolerability, efficacy, and safety of tri-weekly irinotecan plus S-1 (IRIS) and weekly cetuximab in patients with metastatic colorectal cancer (mCRC).
Methods
The main eligibility criteria were RAS wild-type mCRC with no prior chemotherapy. S-1 was given orally at a dose of 40 mg/m2 (40–60 mg) twice for 2 weeks, followed by a 1-week rest. Irinotecan was given on day 1 of each cycle at a dose of 150 mg/m2. Cetuximab was administered on days 1 (400 mg/m2), 8 (250 mg/m2), and 15 (250 mg/m2), and then once weekly (250 mg/m2) thereafter. A standard 3 + 3 phase I dose de-escalation design was used to determine the maximum tolerated dose and the recommended dose (RD) of irinotecan. The primary end point of the Phase II study was overall response rate (ORR).
Results
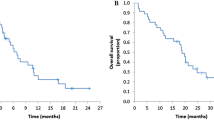
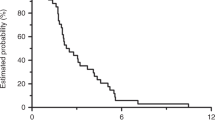
Between December 2014 and September 2017, 4 and 54 patients were enrolled in phase I and phase II studies, respectively. No dose-limiting toxicity was observed in the phase I study, and the RD of irinotecan was 150 mg/m2. In the phase II study, the ORR was 56.9% (90% confidence interval 44.4%–68.7%). The safety profile revealed that the most common grade 3/4 adverse events were neutropenia (31.4%), appetite loss (27.5%), hypokalemia (11.8%), and diarrhea (11.8%). Grade 3/4 hand–foot skin syndrome occurred in nine patients (9.8%).
Conclusion
This study showed that the efficacy and safety of IRIS combined with cetuximab were comparable to those for other first-line treatments. This regimen is a good candidate for first-line treatment of RAS wild-type mCRC.



Similar content being viewed by others
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
The Global Cancer Observatory, International Agency for Research on Cancer 2018. https://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact-sheet.pdf. Accessed 5 Aug 2019
Services CfCCaI (2018) Projected Cancer Statistics. https://ganjoho.jp/en/public/statistics/short_pred.html. Accessed 23 Oct 2018
Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kinugasa Y, Murofushi K, Nakajima TE, Oka S, Tanaka T, Taniguchi H, Tsuji A, Uehara K, Ueno H, Yamanaka T, Yamazaki K, Yoshida M, Yoshino T, Itabashi M, Sakamaki K, Sano K, Shimada Y, Tanaka S, Uetake H, Yamaguchi S, Yamaguchi N, Kobayashi H, Matsuda K, Kotake K, Sugihara K (2019) Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol 15(10):019–01485
Shida D, Ahiko Y, Tanabe T, Yoshida T, Tsukamoto S, Ochiai H, Takashima A, Boku N, Kanemitsu Y (2018) Shorter survival in adolescent and young adult patients, compared to adult patients, with stage IV colorectal cancer in Japan. BMC Cancer 18(1):334. https://doi.org/10.1186/s12885-018-4241-9
Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A (2004) FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 22(2):229–237. https://doi.org/10.1200/JCO.2004.1205.1113(Epub 2003 Dec 1202)
Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzen F, Cassidy J (2008) Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 26(12):2013–2019. https://doi.org/10.1200/JCO.2007.14.9930
Sobrero A, Ackland S, Clarke S, Perez-Carrion R, Chiara S, Gapski J, Mainwaring P, Langer B, Young S, Investigators AT (2009) Phase IV study of bevacizumab in combination with infusional fluorouracil, leucovorin and irinotecan (FOLFIRI) in first-line metastatic colorectal cancer. Oncology 77(2):113–119. https://doi.org/10.1159/000229787
Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D'Haens G, Pinter T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360(14):1408–1417. https://doi.org/10.1056/NEJMoa0805019
Yamada Y, Denda T, Gamoh M, Iwanaga I, Yuki S, Shimodaira H, Nakamura M, Yamaguchi T, Ohori H, Kobayashi K, Tsuda M, Kobayashi Y, Miyamoto Y, Kotake M, Shimada K, Sato A, Morita S, Takahashi S, Komatsu Y, Ishioka C (2018) S-1 and irinotecan plus bevacizumab versus mFOLFOX6 or CapeOX plus bevacizumab as first-line treatment in patients with metastatic colorectal cancer (TRICOLORE): a randomized, open-label, phase III, noninferiority trial. Ann Oncol 29(3):624–631. https://doi.org/10.1093/annonc/mdx1816
Arkenau HT, Arnold D, Cassidy J, Diaz-Rubio E, Douillard JY, Hochster H, Martoni A, Grothey A, Hinke A, Schmiegel W, Schmoll HJ, Porschen R (2008) Efficacy of oxaliplatin plus capecitabine or infusional fluorouracil/leucovorin in patients with metastatic colorectal cancer: a pooled analysis of randomized trials. J Clin Oncol 26(36):5910–5917. https://doi.org/10.1200/JCO.2008.5916.7759(Epub 2008 Nov 5917)
Cassidy J, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Rittweger K, Gilberg F, Saltz L (2011) XELOX vs FOLFOX-4 as first-line therapy for metastatic colorectal cancer: NO16966 updated results. Br J Cancer 105(1):58–64. https://doi.org/10.1038/bjc.2011.1201(Epub 2011 Jun 1014)
Tyagi P, Grothey A (2006) Commentary on a phase III trial of bevacizumab plus XELOX or FOLFOX4 for first-line treatment of metastatic colorectal cancer: the NO16966 trial. Clin Colorectal Cancer 6(4):261–264. https://doi.org/10.3816/CCC.2006.n.3044
Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde CJ, Balmana J, Regula J, Nagtegaal ID, Beets-Tan RG, Arnold D, Ciardiello F, Hoff P, Kerr D, Kohne CH, Labianca R, Price T, Scheithauer W, Sobrero A, Tabernero J, Aderka D, Barroso S, Bodoky G, Douillard JY, El Ghazaly H, Gallardo J, Garin A, Glynne-Jones R, Jordan K, Meshcheryakov A, Papamichail D, Pfeiffer P, Souglakos I, Turhal S, Cervantes A (2012) ESMO Consensus Guidelines for management of patients with colon and rectal cancer: a personalized approach to clinical decision making. Ann Oncol 23(10):2479–2516. https://doi.org/10.1093/annonc/mds2236
Benson AB 3rd, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, Cooper HS, Engstrom PF, Enzinger PC, Fakih MG, Fenton MJ, Fuchs CS, Grem JL, Hunt S, Kamel A, Leong LA, Lin E, May KS, Mulcahy MF, Murphy K, Rohren E, Ryan DP, Saltz L, Sharma S, Shibata D, Skibber JM, Small W Jr, Sofocleous CT, Venook AP, Willett CG, Gregory KM, Freedman-Cass DA (2013) Metastatic colon cancer, version 3.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 11(2):141–152. https://doi.org/10.6004/jnccn.2013.0022(quiz 152)
Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, Ishihara S, Ishiguro M, Kanemitsu Y, Kokudo N, Muro K, Ochiai A, Oguchi M, Ohkura Y, Saito Y, Sakai Y, Ueno H, Yoshino T, Boku N, Fujimori T, Koinuma N, Morita T, Nishimura G, Sakata Y, Takahashi K, Tsuruta O, Yamaguchi T, Yoshida M, Yamaguchi N, Kotake K, Sugihara K, Japanese Society for Cancer of the C, Rectum (2015) Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 20(2):207–239. https://doi.org/10.1007/s10147-015-0801-z
Yamazaki K, Nagase M, Tamagawa H, Ueda S, Tamura T, Murata K, Eguchi Nakajima T, Baba E, Tsuda M, Moriwaki T, Esaki T, Tsuji Y, Muro K, Taira K, Denda T, Funai S, Shinozaki K, Yamashita H, Sugimoto N, Okuno T, Nishina T, Umeki M, Kurimoto T, Takayama T, Tsuji A, Yoshida M, Hosokawa A, Shibata Y, Suyama K, Okabe M, Suzuki K, Seki N, Kawakami K, Sato M, Fujikawa K, Hirashima T, Shimura T, Taku K, Otsuji T, Tamura F, Shinozaki E, Nakashima K, Hara H, Tsushima T, Ando M, Morita S, Boku N, Hyodo I (2016) Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (WJOG4407G). Ann Oncol 27(8):1539–1546. https://doi.org/10.1093/annonc/mdw1206(Epub 2016 May 1513)
Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F, de Gramont A (2009) Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 27(19):3109–3116. https://doi.org/10.1200/JCO.2008.20.6771
Moosmann N, von Weikersthal LF, Vehling-Kaiser U, Stauch M, Hass HG, Dietzfelbinger H, Oruzio D, Klein S, Zellmann K, Decker T, Schulze M, Abenhardt W, Puchtler G, Kappauf H, Mittermuller J, Haberl C, Schalhorn A, Jung A, Stintzing S, Heinemann V (2011) Cetuximab plus capecitabine and irinotecan compared with cetuximab plus capecitabine and oxaliplatin as first-line treatment for patients with metastatic colorectal cancer: AIO KRK-0104–a randomized trial of the German AIO CRC study group. J Clin Oncol 29(8):1050–1058. https://doi.org/10.1200/JCO.2010.1031.1936(Epub 2011 Feb 1057)
Souglakos J, Ziras N, Kakolyris S, Boukovinas I, Kentepozidis N, Makrantonakis P, Xynogalos S, Christophyllakis C, Kouroussis C, Vamvakas L, Georgoulias V, Polyzos A (2012) Randomised phase-II trial of CAPIRI (capecitabine, irinotecan) plus bevacizumab vs FOLFIRI (folinic acid, 5-fluorouracil, irinotecan) plus bevacizumab as first-line treatment of patients with unresectable/metastatic colorectal cancer (mCRC). Br J Cancer 106(3):453–459. https://doi.org/10.1038/bjc.2011.1594(Epub 2012 Jan 1012)
Patt YZ, Lee FC, Liebmann JE, Diamandidis D, Eckhardt SG, Javle M, Justice GR, Keiser W, Salvatore JR, Bexon A, Lin E (2007) Capecitabine plus 3-weekly irinotecan (XELIRI regimen) as first-line chemotherapy for metastatic colorectal cancer: phase II trial results. Am J Clin Oncol 30(4):350–357. https://doi.org/10.1097/COC.1090b1013e31804b31840bb
Schmiegel W, Reinacher-Schick A, Arnold D, Kubicka S, Freier W, Dietrich G, Geissler M, Hegewisch-Becker S, Tannapfel A, Pohl M, Hinke A, Schmoll HJ, Graeven U (2013) Capecitabine/irinotecan or capecitabine/oxaliplatin in combination with bevacizumab is effective and safe as first-line therapy for metastatic colorectal cancer: a randomized phase II study of the AIO colorectal study group. Ann Oncol 24(6):1580–1587. https://doi.org/10.1093/annonc/mdt1028(Epub 2013 Mar 1584)
Shirasaka T, Nakano K, Takechi T, Satake H, Uchida J, Fujioka A, Saito H, Okabe H, Oyama K, Takeda S, Unemi N, Fukushima M (1996) Antitumor activity of 1 M tegafur-0.4 M 5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against human colon carcinoma orthotopically implanted into nude rats. Cancer Res 56(11):2602–2606
Muro K, Boku N, Shimada Y, Tsuji A, Sameshima S, Baba H, Satoh T, Denda T, Ina K, Nishina T, Yamaguchi K, Takiuchi H, Esaki T, Tokunaga S, Kuwano H, Komatsu Y, Watanabe M, Hyodo I, Morita S, Sugihara K (2010) Irinotecan plus S-1 (IRIS) versus fluorouracil and folinic acid plus irinotecan (FOLFIRI) as second-line chemotherapy for metastatic colorectal cancer: a randomised phase 2/3 non-inferiority study (FIRIS study). Lancet Oncol 11(9):853–860. https://doi.org/10.1016/S1470-2045(1010)70181-70189(Epub 72010 Aug 70112)
Yamada Y, Yamaguchi T, Matsumoto H, Ichikawa Y, Goto A, Kato K, Hamaguchi T, Shimada Y (2012) Phase II study of oral S-1 with irinotecan and bevacizumab (SIRB) as first-line therapy for patients with metastatic colorectal cancer. Invest New Drugs 30(4):1690–1696. https://doi.org/10.1007/s10637-011-9743-0
Hong YS, Park YS, Lim HY, Lee J, Kim TW, Kim KP, Kim SY, Baek JY, Kim JH, Lee KW, Chung IJ, Cho SH, Lee KH, Shin SJ, Kang HJ, Shin DB, Jo SJ, Lee JW (2012) S-1 plus oxaliplatin versus capecitabine plus oxaliplatin for first-line treatment of patients with metastatic colorectal cancer: a randomised, non-inferiority phase 3 trial. Lancet Oncol 13(11):1125–1132. https://doi.org/10.1016/S1470-2045(1112)70363-70367(Epub 72012 Oct 70310)
Kim ST, Hong YS, Lim HY, Lee J, Kim TW, Kim KP, Kim SY, Baek JY, Kim JH, Lee KW, Chung IJ, Cho SH, Lee KH, Shin SJ, Kang HJ, Shin DB, Lee JW, Jo SJ, Park YS (2014) S-1 plus oxaliplatin versus capecitabine plus oxaliplatin for the first-line treatment of patients with metastatic colorectal cancer: updated results from a phase 3 trial. BMC Cancer 14:883. https://doi.org/10.1186/1471-2407-1114-1883
Miyamoto Y, Tsuji A, Tanioka H, Maekawa S, Kawanaka H, Kitazono M, Oki E, Emi Y, Murakami H, Ogata Y, Saeki H, Shimokawa M, Natsugoe S, Akagi Y, Baba H, Maehara Y (2016) S-1 and irinotecan plus bevacizumab as second-line chemotherapy for patients with oxaliplatin-refractory metastatic colorectal cancer: a multicenter phase II study in Japan (KSCC1102). Int J Clin Oncol 21(4):705–712. https://doi.org/10.1007/s10147-015-0943-z
Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL, Kay E, Mitchell JK, Madi A, Jasani B, James MD, Bridgewater J, Kennedy MJ, Claes B, Lambrechts D, Kaplan R, Cheadle JP (2011) Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 377(9783):2103–2114. https://doi.org/10.1016/S0140-6736(2111)60613-60612(Epub 62011 Jun 60615)
Cartwright T, Lopez T, Vukelja SJ, Encarnacion C, Boehm KA, Asmar L (2005) Results of a phase II open-label study of capecitabine in combination with irinotecan as first-line treatment for metastatic colorectal cancer. Clin Colorectal Cancer 5(1):50–56. https://doi.org/10.3816/CCC.2005.n.016
Koopman M, Antonini NF, Douma J, Wals J, Honkoop AH, Erdkamp FLG, de Jong RS, Rodenburg CJ, Vreugdenhil G, Loosveld OJL, van Bochove A, Sinnige HAM, Creemers G-JM, Tesselaar MET, Slee PHTJ, Werter MJBP, Mol L, Dalesio O, Punt CJA (2007) Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): a phase III randomised controlled trial. The Lancet 370(9582):135–142. https://doi.org/10.1016/s0140-6736(07)61086-1
Cartwright T, Kuefler P, Cohn A, Hyman W, Berger M, Richards D, Vukelja S, Nugent JE, Ruxer RL Jr, Boehm KA, Asmar L (2008) Results of a phase II trial of cetuximab plus capecitabine/irinotecan as first-line therapy for patients with advanced and/or metastatic colorectal cancer. Clin Colorectal Cancer 7(6):390–397. https://doi.org/10.3816/CCC.2008.n.052
Acknowledgements
We sincerely thank the participating patients and their families. We are indebted to the physicians and all other medical staff. We also thank Ms. Sakamoto and the staff at the KSCC (Kyushu Study Group of Clinical Cacner) and CReS Kyushu for their excellent data collection and management, secretarial assistance, and support. Finally, we thank James P. Mahaffey, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/) for editing a draft of this manuscript. This work was supported by the CReS Kyushu with funding from Merck Biopharma Co., Ltd., an affiliate of Merck KGaA, Darmstadt, Germany under a research contract.
Funding
This work was supported by the CReS Kyushu with funding from Merck Serono Co., Ltd., an affiliate of Merck KGaA, Darmstadt, Germany under a research contract.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Eiji Oki received lecture fee from Merck Biopharma Co., Ltd., Bayer Yakuhin Japan, Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Eli Lilly, Yakult Honsha Co., Ltd., and Takeda Pharmaceutical Co., Ltd. Akitaka Makiyama reports personal fees from Lily, personal fees from Chugai, personal fees from Takeda, outside the submitted work. Masahito Kotaka reports lecturefee from Chugai Pharmaceutical Co., Ltd., Yakult Honsha Co., Ltd., Takeda Pharmaceutical Company Limited, Merck Biopharma Co., Ltd., and Taiho Pharmaceutical Co., Ltd., outside the submitted work. Hideo Baba reports grants, personal fees and non-financial support from Taiho Pharmaceutical Co., Ltd., grants and non-financial support from Merck Biopharma Co., Ltd., during the conduct of the study; grants, personal fees and non-financial support from Ono Pharmaceutical Co., Ltd., grants, personal fees and non-financial support from Eli Lilly Japan K.K., grants and personal fees from Takeda Pharmaceutical Co., Ltd., grants and personal fees from Chugai Pharmaceutical Co., Ltd., grants from Shionogi & Co., Ltd., grants from Covidien Japan Inc., grants and non-financial support from Yakult Honsha Co.,Ltd., grants from Shin Nippon Biomedical Laboratories, Ltd., grants from Novartis-pharma K.K., grants from Toyama Chemical Co., Ltd., grants and non-financial support from Johnson & Johnson K.K., outside the submitted work. Other authors have no conflict of interest.
Ethics approval (include appropriate approvals or waivers)
This study was conducted in accordance with the Declaration of Helsinki and Ethical Guidelines for Clinical Studies in Japan, and the protocol was approved by the institutional review boards of all participating medical institutions. This study was registered in the UMIN clinical trials registry. (UMIN-CTR number UMIN000023329).
Informed consent
The informed consent form stated that the results would be published after completion of the study. Consent of the family is not required at the time of article publication according to Japanese ethical guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Samura, H., Oki, E., Okumura, H. et al. A phase I/II study of S-1 and irinotecan (IRIS) combined with cetuximab in patients with RAS wild-type metastatic colorectal cancer (KSCC1401). Cancer Chemother Pharmacol 86, 285–294 (2020). https://doi.org/10.1007/s00280-020-04108-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-020-04108-x