Abstract
Background
Several studies assessed the association of docetaxel dose intensity (DI) and efficacy in metastatic castrate-resistant prostate cancer (mCRPC) patients with contradicting conclusions. In this retrospective analysis, we will assess whether the docetaxel DI used in patients with metastatic castrate-sensitive prostate cancer (mCSPC) is associated with overall survival (OS).
Methods
All patients with mCSPC treated at The Ottawa Hospital Cancer Centre that received docetaxel chemotherapy between June 2014 and September 2017 were identified. The association between relative dose intensity (RDI) and OS was assessed using univariate and multivariable Cox model adjusting for age, Gleason score, burden of disease, visceral involvement, de novo metastases and baseline prostate-specific antigen (PSA).
Results
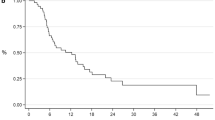
Eighty-one patients were included in the analysis. Only 35 patients (43%) were able to complete the planned treatment with a RDI of at least 90%. On a univariate analysis, higher RDI and number of cycles of docetaxel received were associated with longer OS. For every 10% decrease in RDI, the risk of death increased by 23% (HR 1.23, 95% CI 1.09–1.4, P = 0.001). For every increment of one cycle (and up to six), the risk of death decreased by 27% (HR 0.73, 95% CI 0.61–0.88, P = 0.001). On multivariate analysis, reduced RDI was the only predictor significantly associated with OS (HR 1.18, 95% CI 1.02–1.36, P = 0.026).
Conclusions
Our study suggests that in mCSPC, reduced docetaxel RDI is associated with shorter survival. Unnecessary dose reductions, treatment delays and early discontinuation should be avoided. Granulocyte colony-stimulating factor may be considered to maintain standard DI.
Similar content being viewed by others
References
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN et al (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351:1502–1512. https://doi.org/10.1056/NEJMoa040720
Sweeney CJ, Chen Y-H, Carducci M, Liu G, Jarrard DF, Eisenberger M et al (2015) Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med 373:737–746. https://doi.org/10.1056/NEJMoa1503747
James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR et al (2016) Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet Lond Engl 387:1163–1177. https://doi.org/10.1016/S0140-6736(15)01037-5
Kawatkar AA, Farias AJ, Chao C, Chen W, Barron R, Vogl FD et al (2017) Hospitalizations, outcomes, and management costs of febrile neutropenia in patients from a managed care population. Support Care Cancer 25:2787–2795. https://doi.org/10.1007/s00520-017-3692-x
Tsao C-K, Galsky MD, Oh WK (2016) Docetaxel for metastatic hormone-sensitive prostate cancer: urgent need to minimize the risk of neutropenic fever. Eur Urol 70:707–708. https://doi.org/10.1016/j.eururo.2016.06.041
Lavoie J-M, Zou K, Khalaf D, Eigl BJ, Kollmannsberger CK, Vergidis J et al (2019) Clinical effectiveness of docetaxel for castration-sensitive prostate cancer in a real-world population-based analysis. Prostate 79:281–287. https://doi.org/10.1002/pros.23733
Hamid AA, Willson K, Vincent AD, Tamjid B, Lee M, Bergin A et al (2018) Risk of febrile neutropenia and early treatment cessation in men receiving standard and dose-reduced 3-weekly docetaxel for metastatic castration-resistant prostate cancer. Asia Pac J Clin Oncol 14:e399–404. https://doi.org/10.1111/ajco.12840
Kamiya N, Suzuki H, Ueda T, Sato N, Nakatsu H, Mikami K et al (2014) Clinical outcomes by relative docetaxel dose and dose intensity as chemotherapy for Japanese patients with castration-resistant prostate cancer: a retrospective multi-institutional collaborative study. Int J Clin Oncol 19:157–164. https://doi.org/10.1007/s10147-012-0510-9
Kita Y, Shimizu Y, Inoue T, Kamba T, Yoshimura K, Ogawa O (2013) Reduced-dose docetaxel for castration-resistant prostate cancer has no inferior impact on overall survival in Japanese patients. Int J Clin Oncol 18:718–723. https://doi.org/10.1007/s10147-012-0443-3
Lyman GH (2009) Impact of chemotherapy dose intensity on cancer patient outcomes. J Natl Compr Canc Netw 7:99–108. https://doi.org/10.6004/jnccn.2009.0009
Foote M (1998) The importance of planned dose of chemotherapy on time: do we need to change our clinical practice? Oncologist 3:365–368
Prasad N, Bakshi A, Deshmukh C, Hingmire S, Ranade A, Parikh P (2012) Importance of dose intensity in treatment of advanced non-small cell lung cancer in the elderly. South Asian J Cancer 1:9–15. https://doi.org/10.4103/2278-330X.96494
Kyriakopoulos CE, Chen YH, Carducci MA, Liu G, Jarrard DF, Hahn NM et al (2018) Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol 36(11):1080–1087. https://doi.org/10.1200/JCO.2017.75.3657
Vogel CL, Wojtukiewicz MZ, Carroll RR, Tjulandin SA, Barajas-Figueroa LJ, Wiens BL et al (2005) First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol 23:1178–1184. https://doi.org/10.1200/JCO.2005.09.102
Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N et al (1990) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer Oxf Engl 2011(47):8–32. https://doi.org/10.1016/j.ejca.2010.10.013
Smith TJ, Bohlke K, Lyman GH, Carson KR, Crawford J, Cross SJ et al (2015) Recommendations for the use of WBC growth factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 33:3199–3212. https://doi.org/10.1200/JCO.2015.62.3488
Kushnir I, Koczka K, Ong M, Canil C, Bossé D, Sabri E et al (2019) The timing of docetaxel initiation in metastatic castrate-sensitive prostate cancer and the rate of chemotherapy-induced toxicity. Med Oncol Northwood Lond Engl 36:18. https://doi.org/10.1007/s12032-018-1238-9
Mahil J, Hughes C, Patel K, Lyons J, Elliott PA, Choudhury A et al (2016) Febrile neutropenia rates in men treated with docetaxel chemotherapy for metastatic hormone-sensitive prostate cancer. Clin Oncol 28:612. https://doi.org/10.1016/j.clon.2016.06.006
Griggs JJ, Mangu PB, Anderson H, Balaban EP, Dignam JJ, Hryniuk WM et al (2012) Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 30:1553–1561. https://doi.org/10.1200/JCO.2011.39.9436
Hourdequin KC, Schpero WL, McKenna DR, Piazik BL, Larson RJ (2013) Toxic effect of chemotherapy dosing using actual body weight in obese versus normal-weight patients: a systematic review and meta-analysis. Ann Oncol 24:2952–2962. https://doi.org/10.1093/annonc/mdt294
Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY et al (2017) Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med 377:352–360. https://doi.org/10.1056/NEJMoa1704174
Chi KN, Agarwal N, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, Given R et al (2019) Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. https://doi.org/10.1056/NEJMoa1903307
Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S et al (2019) Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. https://doi.org/10.1056/NEJMoa1903835
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
IK declares a research grant from Astellas (unrelated to the current study). Stock ownership—Teva Pharmaceutical. Honoraria from Merck Sharp & Dohme (MSD), Bristol-Myers Squibb and Janssen. Consulting or advisory role from Merck Sharp & Dohme (MSD). MO declares honoraria from Jannsen and Astellas; Travel expenses from Jannsen. CC declares consulting or advisory role for Merck, EMD Serono, Pfizer, Novartis, Bayer, Bristol-Myers Squibb and Astra Zeneca; Honoraria from Janssen; Research funding (unrelated to the current study) from Eisai, Astellas, Janssen, Astra Zeneca and Roche; Travel expenses from Sanofi Grenzyme and Amgen. DB declares consulting or advisory role for Bristol-Myers Squibb (Inst), Pfizer, AbbVie; Honoraria from AstraZeneca, Ipsen, Amgen and Janssen. NMR declares consulting or advisory role for Astellas Pharma and Pfizer; Honoraria from AstraZeneca, Eisai, Ipsen, Merck, Novartis and Roche. RM and KK declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
For this retrospective study, formal consent was not required by the Ottawa Health Science Network Research Ethics Board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kushnir, I., Mallick, R., Ong, M. et al. Docetaxel dose-intensity effect on overall survival in patients with metastatic castrate-sensitive prostate cancer. Cancer Chemother Pharmacol 85, 863–868 (2020). https://doi.org/10.1007/s00280-020-04063-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-020-04063-7