Abstract
Purpose
High-dose methotrexate (HD-MTX) is widely used in pediatric and adult oncology treatment regimens. This study aimed to develop a population pharmacokinetic model to characterize pediatric and adult MTX exposure across various disease types and dosing regimens, and to evaluate exposure–toxicity relationships.
Methods
MTX pharmacokinetic data from pediatric and adult patients were collected. A population pharmacokinetic model was developed to determine the effects of age, liver function, renal function, and demographics on MTX disposition. The final model was used in Monte Carlo simulations to generate expected exposures for different dosing regimens. The association of toxicity, determined through chart review, and MTX area under the curve (AUC) was modeled using logistic regression.
Results
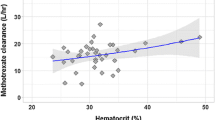
The analysis included 5116 MTX concentrations from 320 patients (135 adult, age 19–79 years; 185 pediatric, age 0.6–19 years). Estimated glomerular filtration rate (eGFR) and treatment cycle number were independent predictors of clearance (CL). CL varied 2.1-fold over the range of study eGFR values and increased 14% for treatment cycle numbers greater than 7. Higher MTX AUC was associated with higher risk of nephrotoxicity in adults, and neurotoxicity and hepatotoxicity in pediatrics.
Conclusions
This study represents one of the most comprehensive evaluations of HD-MTX PK across a wide range of ages and disease types. After accounting for differences in renal function, age did not impact CL, although toxicity patterns differed by age. The model allows for early identification of patients with slowed MTX clearance and at higher risk of toxicity.



Similar content being viewed by others
References
Goldman ID, Matherly LH (1985) The cellular pharmacology of methotrexate. Pharmacol Ther 28(1):77–102
Ackland SP, Schilsky RL (1987) High-dose methotrexate: a critical reappraisal. J Clin Oncol 5(12):2017–2031. https://doi.org/10.1200/JCO.1987.5.12.2017
Howard SC, McCormick J, Pui CH, Buddington RK, Harvey RD (2016) Preventing and managing toxicities of high-dose methotrexate. Oncologist 21(12):1471–1482. https://doi.org/10.1634/theoncologist.2015-0164
Widemann BC, Adamson PC (2006) Understanding and managing methotrexate nephrotoxicity. Oncologist 11(6):694–703. https://doi.org/10.1634/theoncologist.11-6-694
Widemann BC, Balis FM, Kim A, Boron M, Jayaprakash N, Shalabi A, O’Brien M, Eby M, Cole DE, Murphy RF, Fox E, Ivy P, Adamson PC (2010) Glucarpidase, leucovorin, and thymidine for high-dose methotrexate-induced renal dysfunction: clinical and pharmacologic factors affecting outcome. J Clin Oncol 28(25):3979–3986. https://doi.org/10.1200/JCO.2009.25.4540
Monjanel S, Rigault JP, Cano JP, Carcassonne Y, Favre R (1979) High-dose methotrexate: preliminary evaluation of a pharmacokinetic approach. Cancer Chemother Pharmacol 3(3):189–196
Abelson HT, Fosburg MT, Beardsley GP, Goorin AM, Gorka C, Link M, Link D (1983) Methotrexate-induced renal impairment: clinical studies and rescue from systemic toxicity with high-dose leucovorin and thymidine. J Clin Oncol 1(3):208–216. https://doi.org/10.1200/JCO.1983.1.3.208
Widemann BC, Balis FM, Kempf-Bielack B, Bielack S, Pratt CB, Ferrari S, Bacci G, Craft AW, Adamson PC (2004) High-dose methotrexate-induced nephrotoxicity in patients with osteosarcoma. Cancer 100(10):2222–2232. https://doi.org/10.1002/cncr.20255
Green JM (2012) Glucarpidase to combat toxic levels of methotrexate in patients. Ther Clin Risk Manag 8:403–413. https://doi.org/10.2147/TCRM.S30135
Ramsey LB, Balis FM, O’Brien MM, Schmiegelow K, Pauley JL, Bleyer A, Widemann BC, Askenazi D, Bergeron S, Shirali A, Schwartz S, Vinks AA, Heldrup J (2018) Consensus guideline for use of glucarpidase in patients with high-dose methotrexate induced acute kidney injury and delayed methotrexate clearance. Oncologist 23(1):52–61. https://doi.org/10.1634/theoncologist.2017-0243
Glucarpidase Package Insert (2019) https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/125327lbl.pdf. Accessed May 7 2019
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16(1):31–41. https://doi.org/10.1159/000180580
Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20(3):629–637. https://doi.org/10.1681/ASN.2008030287
Zhang W, Zhang Q, Tian X, Zhao H, Lu W, Zhen J, Niu X (2015) Population pharmacokinetics of high-dose methotrexate after intravenous administration in Chinese osteosarcoma patients from a single institution. Chin Med J (Engl) 128(1):111–118. https://doi.org/10.4103/0366-6999.147829
Min Y, Qiang F, Peng L, Zhu Z (2009) High dose methotrexate population pharmacokinetics and Bayesian estimation in patients with lymphoid malignancy. Biopharm Drug Dispos 30(8):437–447. https://doi.org/10.1002/bdd.678
Johansson AM, Hill N, Perisoglou M, Whelan J, Karlsson MO, Standing JF (2011) A population pharmacokinetic/pharmacodynamic model of methotrexate and mucositis scores in osteosarcoma. Ther Drug Monit 33(6):711–718. https://doi.org/10.1097/FTD.0b013e31823615e1
Comandone A, Passera R, Boglione A, Tagini V, Ferrari S, Cattel L (2005) High dose methotrexate in adult patients with osteosarcoma: clinical and pharmacokinetic results. Acta Oncol 44(4):406–411. https://doi.org/10.1080/02841860510029770
Mei S, Li X, Jiang X, Yu K, Lin S, Zhao Z (2018) Population pharmacokinetics of high-dose methotrexate in patients with primary central nervous system lymphoma. J Pharm Sci 107(5):1454–1460. https://doi.org/10.1016/j.xphs.2018.01.004
Fukuhara K, Ikawa K, Morikawa N, Kumagai K (2008) Population pharmacokinetics of high-dose methotrexate in Japanese adult patients with malignancies: a concurrent analysis of the serum and urine concentration data. J Clin Pharm Ther 33(6):677–684. https://doi.org/10.1111/j.1365-2710.2008.00966.x
Dupuis C, Mercier C, Yang C, Monjanel-Mouterde S, Ciccolini J, Fanciullino R, Pourroy B, Deville JL, Duffaud F, Bagarry-Liegey D, Durand A, Iliadis A, Favre R (2008) High-dose methotrexate in adults with osteosarcoma: a population pharmacokinetics study and validation of a new limited sampling strategy. Anticancer Drugs 19(3):267–273
Hui KH, Chu HM, Fong PS, Cheng WTF, Lam TN (2019) Population pharmacokinetic study and individual dose adjustments of high-dose methotrexate in Chinese pediatric patients with acute lymphoblastic leukemia or osteosarcoma. J Clin Pharmacol 59(4):566–577. https://doi.org/10.1002/jcph.1349
Nader A, Zahran N, Alshammaa A, Altaweel H, Kassem N, Wilby KJ (2017) Population pharmacokinetics of intravenous methotrexate in patients with hematological malignancies: utilization of routine clinical monitoring parameters. Eur J Drug Metab Pharmacokinet 42(2):221–228. https://doi.org/10.1007/s13318-016-0338-1
Relling MV, Fairclough D, Ayers D, Crom WR, Rodman JH, Pui CH, Evans WE (1994) Patient characteristics associated with high-risk methotrexate concentrations and toxicity. J Clin Oncol 12(8):1667–1672. https://doi.org/10.1200/JCO.1994.12.8.1667
Widemann BC, Balis FM, Murphy RF, Sorensen JM, Montello MJ, O’Brien M, Adamson PC (1997) Carboxypeptidase-G2, thymidine, and leucovorin rescue in cancer patients with methotrexate-induced renal dysfunction. J Clin Oncol 15(5):2125–2134. https://doi.org/10.1200/JCO.1997.15.5.2125
Tsurusawa M, Gosho M, Mori T, Mitsui T, Sunami S, Kobayashi R, Fukano R, Tanaka F, Fujita N, Inada H, Koh K, Takimoto T, Saito A, Fujimoto J, Nakazawa A, Horibe K, Lymphoma committee of the Japanese Pediatric Leukemia/lymphoma Study G (2015) Statistical analysis of relation between plasma methotrexate concentration and toxicity in high-dose methotrexate therapy of childhood nonHodgkin lymphoma. Pediatr Blood Cancer 62(2):279–284. https://doi.org/10.1002/pbc.25305
Rask C, Albertioni F, Bentzen SM, Schroeder H, Peterson C (1998) Clinical and pharmacokinetic risk factors for high-dose methotrexate-induced toxicity in children with acute lymphoblastic leukemia–a logistic regression analysis. Acta Oncol 37(3):277–284
Buchen S, Ngampolo D, Melton RG, Hasan C, Zoubek A, Henze G, Bode U, Fleischhack G (2005) Carboxypeptidase G2 rescue in patients with methotrexate intoxication and renal failure. Br J Cancer 92(3):480–487. https://doi.org/10.1038/sj.bjc.6602337
Bhojwani D, Sabin ND, Pei D, Yang JJ, Khan RB, Panetta JC, Krull KR, Inaba H, Rubnitz JE, Metzger ML, Howard SC, Ribeiro RC, Cheng C, Reddick WE, Jeha S, Sandlund JT, Evans WE, Pui CH, Relling MV (2014) Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol 32(9):949–959. https://doi.org/10.1200/JCO.2013.53.0808
Hegyi M, Gulacsi A, Csagoly E, Csordas K, Eipel OT, Erdelyi DJ, Muller J, Nemes K, Lautner-Csorba O, Kovacs GT (2012) Clinical relations of methotrexate pharmacokinetics in the treatment for pediatric osteosarcoma. J Cancer Res Clin Oncol 138(10):1697–1702. https://doi.org/10.1007/s00432-012-1214-2
Joerger M, Huitema AD, Krahenbuhl S, Schellens JH, Cerny T, Reni M, Zucca E, Cavalli F, Ferreri AJ (2010) Methotrexate area under the curve is an important outcome predictor in patients with primary CNS lymphoma: a pharmacokinetic-pharmacodynamic analysis from the IELSG no. 20 trial. Br J Cancer 102(4):673–677. https://doi.org/10.1038/sj.bjc.6605559
Acknowledgements
The authors would like to thank Dr. Sam Martinez, Dr. Jeremiah Momper, Dr. Lawrence Alejandro, and Dr. Don Barkauskas for help with the project. Funding support was provided by a Research in Pediatric and Developmental Pharmacology NIH grant (1U54HD090259-01, Dr. Capparelli).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Capparelli serves on the data safety and monitoring board for Melinta Pharmaceuticals, Cempra Pharmaceuticals and The Medicines Company.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kawakatsu, S., Nikanjam, M., Lin, M. et al. Population pharmacokinetic analysis of high-dose methotrexate in pediatric and adult oncology patients. Cancer Chemother Pharmacol 84, 1339–1348 (2019). https://doi.org/10.1007/s00280-019-03966-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-03966-4