Abstract
Purpose
Octreotide SC depot is a novel, ready-to-use formulation administered via a thin needle. In a phase 1 study in healthy volunteers, this formulation provided higher bioavailability of octreotide with faster onset and stronger suppression of IGF-1 in healthy volunteers versus long-acting intramuscular (IM) octreotide. This phase 2 study evaluated the pharmacokinetics, efficacy, and safety of octreotide SC depot in patients with acromegaly and functioning NETs, previously treated with octreotide IM.
Methods
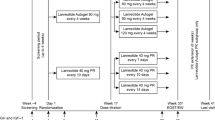
Adult patients with acromegaly or functioning NETs treated for ≥ 2 months with octreotide IM [10/20/30 mg every 4 weeks (q4w)] received the last dose of octreotide IM treatment in study period 0 and were randomized 28 days later to receive octreotide SC depot 10 mg q2w, or 20 mg q4w for 3 months (period 1). The primary objective was to characterize the PK profile of octreotide SC depot after each injection vs PK for octreotide IM (period 0).
Results
Twelve patients were randomized to receive octreotide SC depot 10 mg q2w (acromegaly n = 3; NET n = 1) or 20 mg q4w (acromegaly n = 4; NET n = 4). Plasma levels of octreotide were higher with octreotide SC depot as compared to octreotide IM. Adverse events were reported in 6 and 8 patients during period 0 and period 1, respectively; most common in period 1 were gastrointestinal disorders.
Conclusion
Octreotide SC depot provided higher exposure (AUC) than octreotide IM, maintained biochemical control in patients with acromegaly and symptom control in patients with functioning NETs, and was well tolerated with a safety profile consistent with octreotide IM.
ClinicalTrials.gov identifier
NCT02299089.




Similar content being viewed by others
References
Pavel M, Kidd M, Modlin I (2013) Systemic therapeutic options for carcinoid. Semin Oncol 40(1):84–99. https://doi.org/10.1053/j.seminoncol.2012.11.003
Katznelson L, Laws ER Jr, Melmed S, Molitch ME, Murad MH, Utz A, Wass JA, Endocrine S (2014) Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 99(11):3933–3951. https://doi.org/10.1210/jc.2014-2700
Anthony L, Freda PU (2009) From somatostatin to octreotide LAR: evolution of a somatostatin analogue. Curr Med Res Opin 25(12):2989–2999. https://doi.org/10.1185/03007990903328959
Rinke A, Muller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, Mayer C, Aminossadati B, Pape UF, Blaker M, Harder J, Arnold C, Gress T, Arnold R, Group PS (2009) Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol Off J Am Soc Clin Oncol 27(28):4656–4663. https://doi.org/10.1200/JCO.2009.22.8510
Kulke MH, Shah MH, Benson AB 3rd, Bergsland E, Berlin JD, Blaszkowsky LS, Emerson L, Engstrom PF, Fanta P, Giordano T, Goldner WS, Halfdanarson TR, Heslin MJ, Kandeel F, Kunz PL, Kuvshinoff BW 2nd, Lieu C, Moley JF, Munene G, Pillarisetty VG, Saltz L, Sosa JA, Strosberg JR, Vauthey JN, Wolfgang C, Yao JC, Burns J, Freedman-Cass D, National comprehensive cancer n (2015) Neuroendocrine tumors, version 1.2015. J Natl Compr Canc Netw 13 (1):78–108
Caplin ME, Pavel M, Cwikla JB, Phan AT, Raderer M, Sedlackova E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Blumberg J, Ruszniewski P, Investigators C (2014) Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 371(3):224–233. https://doi.org/10.1056/NEJMoa1316158
Schmid HA (2008) Pasireotide (SOM230): development, mechanism of action and potential applications. Mol Cell Endocrinol 286(1–2):69–74. https://doi.org/10.1016/j.mce.2007.09.006
Sandostatin® LAR Depot Dosing & Administration|HCP (2017) Novartis Pharmaceuticals Corporation, East Hanover. https://www.hcp.novartis.com/products/sandostatin-lar-depot/carcinoid-syndrome/dosing-administration/#mixing-and-administration-guide. Accessed 27 Jun 2017
Tiberg F, Johnsson M (2011) Drug delivery applications of non-lamellar liquid crystalline phases and nanoparticles. J Drug Deliv Sci Technol 21(1):101–109
Tiberg F, Johnsson M, Jankunec M, Barauskas J (2012) Phase behavior, functions, and medical applications of soy phosphatidylcholine and diglyceride lipid compositions. Chem Lett 41(10):1090–1092. https://doi.org/10.1246/cl.2012.1090
Tiberg F, Roberts J, Cervin C, Johnsson M, Sarp S, Tripathi AP, Linden M (2015) Octreotide s.c. depot provides sustained octreotide bioavailability and similar IGF-1 suppression to octreotide LAR in healthy volunteers. Br J Clin Pharmacol 80(3):460–472. https://doi.org/10.1111/bcp.12698
Bosman FTCF, Hruban RH, Theise ND (2010) WHO classification of tumours of the digestive system, vol 3, 4th edn. International Agency for Research on Cancer (IARC), Lyon
Sandostatin® LAR Depot (octreotide acetate for injectable suspension) [prescribing information] (2016) Novartis Pharmaceuticals Corporation, East Hanover. https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/sandostatin_lar.pdf. Accessed 27 Jun 2017
Kvols LK, Oberg KE, O’Dorisio TM, Mohideen P, de Herder WW, Arnold R, Hu K, Zhang Y, Hughes G, Anthony L, Wiedenmann B (2012) Pasireotide (SOM230) shows efficacy and tolerability in the treatment of patients with advanced neuroendocrine tumors refractory or resistant to octreotide LAR: results from a phase II study. Endocr Relat Cancer 19(5):657–666. https://doi.org/10.1530/ERC-11-0367
Wolin EM, Hu K, Hughes G, Bouillaud E, Giannone V, Resendiz KH (2013) Safety, tolerability, pharmacokinetics, and pharmacodynamics of a long-acting release (LAR) formulation of pasireotide (SOM230) in patients with gastroenteropancreatic neuroendocrine tumors: results from a randomized, multicenter, open-label, phase I study. Cancer Chemother Pharmacol 72(2):387–395. https://doi.org/10.1007/s00280-013-2202-1
Buil-Bruna N, Garrido MJ, Dehez M, Manon A, Nguyen TX, Gomez-Panzani EL, Troconiz IF (2016) Population pharmacokinetic analysis of lanreotide autogel/depot in the treatment of neuroendocrine tumors: pooled analysis of four clinical trials. Clin Pharmacokinet 55(4):461–473. https://doi.org/10.1007/s40262-015-0329-4
Hu M, Tomlinson B (2010) Pharmacokinetic evaluation of lanreotide. Expert Opin Drug Metab Toxicol 6(10):1301–1312. https://doi.org/10.1517/17425255.2010.513700
Yang LP, Keating GM (2010) Octreotide long-acting release (LAR): a review of its use in the management of acromegaly. Drugs 70(13):1745–1769. https://doi.org/10.2165/11204510-000000000-00000
Acknowledgements
This study was sponsored by Camurus in collaboration with Novartis. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. We thank all the patients, investigators and nurses who participated in this trial. We thank Mayank Singh, Novartis Healthcare Pvt Ltd for medical editorial assistance with this manuscript.
Funding
This study was sponsored by Camurus in collaboration with Novartis Pharma AG.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MP received honoraria for presentations or advisory board from Novartis, IPSEN, Pfizer, and Lexicon. AC has nothing to disclose. FBC received consulting fees for advisory board from Novartis, Ipsen, Merck Serono, Bayer, and Pfizer; honorarium for speaking from Bayer, HAC, and Genzyme. DH received personal fees from Lexicon Pharma, grants and personal fees from Ipsen Pharma Inc, during the conduct of the study; personal fees from Lexicon Pharma Inc; grants and personal fees from Ipsen Pharma Inc, Novartis Pharma Inc, and Pfizer Pharma Inc, outside the submitted work. HL received consulting fees from Novartis. RP received research grant as principal investigator and co-investigator, and lecturer fee and honoraria from Novartis; research grant as clinical researcher, consulting fees, and honoraria as speaker from Shire; research grant as principal investigator from Ipsen and Pfizer. LT and CD are employees of Novartis. HO and FT are employees of Camurus. DF received consulting fee for lectures or advisory boards, and research funding for preclinical research project from Novartis; received consulting fee for advisory boards, and research funding for preclinical research project from Ipsen.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and applicable local regulations. The study protocol and all amendments were reviewed by the independent ethics committee or institutional review board for each center. All patients provided written informed consent to participate in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pavel, M., Borson-Chazot, F., Cailleux, A. et al. Octreotide SC depot in patients with acromegaly and functioning neuroendocrine tumors: a phase 2, multicenter study. Cancer Chemother Pharmacol 83, 375–385 (2019). https://doi.org/10.1007/s00280-018-3734-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3734-1