Abstract
Purpose
Desensitization is a safe alternative for patients with hypersensitivity reactions (HSRs) to platinum-based chemotherapeutic agents and widely used in real practice by employing stepwise administration of multiple serial dilutions of the culprit drugs. However, its labor-intensive nature has required a simpler protocol that is easier to prepare and perform.
Methods
We performed an observational study of patients with platinum HSR who underwent a new non-dilution one-bag desensitization protocol. Premedication consisted of Montelukast as well as H1 and H2 blockers. The outcomes and safety profiles of a new protocol were assessed.
Results
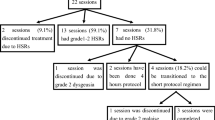
A total of 36 patients were recruited (oxaliplatin 23, carboplatin 9, and cisplatin 4) and the most common grade of HSR presented was grade 2 (61.1%), followed by grade 3 (25%), and grade 1 (13.9%). Of 175 desensitization procedures, all cases were successfully completed in re-administration of culprit chemotherapeutic platinum agents; 146 (83.4%) had no breakthrough reactions (BTRs) while 29 (16.6%) did. Most BTRs were mild reactions (grade 1, 51.7%) or moderate reactions (grade 2, 44.8%) of Brown’s Scale. Although there was one case of asymptomatic mild hypotension (grade 3, 3.5%), categorized as severe reaction, dyspnea, desaturation, and anaphylaxis did not occur. The proportion of severe HSRs was significantly lower than that of initial HSRs (3.5% vs. 25%, P = 0.0167).
Conclusions
The new non-dilution desensitization protocol was safe and effective for re-administration of culprit platinum agents in patients with a history of HSRs. Therefore, this new protocol can be used as an alternative to existing protocols using multiple serial dilutions.


Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the curve
- BTR:
-
Breakthrough reaction
- HSR:
-
Hypersensitivity reaction
References
Dasari S, Tchounwou PB (2014) Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 740:364–378
Kelland L (2007) The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 7(8):573
Miyamoto S, Okada R, Ando K (2015) Platinum hypersensitivity and desensitization. Jpn J Clin Oncol 45(9):795–804
Caiado J, Picard M (2014) Diagnostic tools for hypersensitivity to platinum drugs and taxanes: skin testing, specific IgE, and mast cell/basophil mediators. Curr Allergy Asthma Rep 14(8):1–12
Sliesoraitis S, Chikhale P (2005) Carboplatin hypersensitivity. Int J Gynecol Cancer 15(1):13–18
Comella P, Casaretti R, Sandomenico C, Avallone A, Franco L (2009) Role of oxaliplatin in the treatment of colorectal cancer. Therap Clin Risk Manag 5:229
Markman M, Bookman MA (2000) Second-line treatment of ovarian cancer. The Oncologist 5(1):26–35
Network NCC (2017) NCCN clinical practice guidelines in oncology-ovarian cancer including fallopian tube cancer and primary peritoneal cancer (Version 2.2017)
Castells MC, Tennant NM, Sloane DE, Hsu FI, Barrett NA, Hong DI, Laidlaw TM, Legere HJ, Nallamshetty SN, Palis RI (2008) Hypersensitivity reactions to chemotherapy: outcomes and safety of rapid desensitization in 413 cases. J Allergy Clin Immunol 122(3):574–580
Li Q, Cohn D, Waller A, Backes F, Copeland L, Fowler J, Salani R, O’Malley D (2014) Outpatient rapid 4-step desensitization for gynecologic oncology patients with mild to low-risk, moderate hypersensitivity reactions to carboplatin/cisplatin. Gynecol Oncol 135(1):90–94
Brown SG (2004) Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol 114(2):371–376
Lee C-W, Matulonis UA, Castells MC (2005) Rapid inpatient/outpatient desensitization for chemotherapy hypersensitivity: standard protocol effective in 57 patients for 255 courses. Gynecol Oncol 99(2):393–399
Hesterberg PE, Banerji A, Oren E, Penson RT, Krasner CN, Seiden MV, Wong JT (2009) Risk stratification for desensitization of patients with carboplatin hypersensitivity: clinical presentation and management. J Allergy Clin Immunol 123(6):1262–1267. e1261
Madrigal-Burgaleta R, Berges-Gimeno M, Angel-Pereira D, Ferreiro-Monteagudo R, Guillen-Ponce C, Pueyo C, Gomez de Salazar E, Alvarez-Cuesta E (2013) Hypersensitivity and desensitization to antineoplastic agents: outcomes of 189 procedures with a new short protocol and novel diagnostic tools assessment. Allergy 68(7):853–861
Wong JT, Ling M, Patil S, Banerji A, Long A (2014) Oxaliplatin hypersensitivity: evaluation, implications of skin testing, and desensitization. J Allergy Clin Immunol Pract 2(1):40–45
Nozawa H, Muto Y, Yamada Y (2008) Desensitization to oxaliplatin with two stages of premedication in a patient with metastatic rectal cancer. Clin Therap 30(6):1160–1165
Park H, Lee J, Kim S, Kim S, Park K, Lee C, Kang B, Beom S, Shin S, Jung M (2016) A new practical desensitization protocol for oxaliplatin-induced immediate hypersensitivity reactions: a necessary and useful approach. J Investig Allergol Clin Immunol 26(3):168–176
Confino-Cohen R, Fishman A, Altaras M, Goldberg A (2005) Successful carboplatin desensitization in patients with proven carboplatin allergy. Cancer 104(3):640–643
Cortijo-Cascajares S, Nacle-López I, García-Escobar I, Aguilella-Vizcaíno MJ, Herreros-de-Tejada A, Castro HC-F, Calleja-Hernández M-Á (2013) Effectiveness of oxaliplatin desensitization protocols. Clin Transl Oncol 15(3):219–225
Takase N, Matsumoto K, Onoe T, Kitao A, Tanioka M, Kikukawa Y, Yamaguchi S, Fujiwara K, Negoro S (2015) 4-Step 4-h carboplatin desensitization protocol for patients with gynecological malignancies showing platinum hypersensitivity: a retrospective study. Int J Clin Oncol 20(3):566–573
Okayama T, Ishikawa T, Sugatani K, Yoshida N, Kokura S, Matsuda K, Tsukamoto S, Ihara N, Kuriu Y, Nakanishi M (2015) Hypersensitivity reactions to oxaliplatin: identifying the risk factors and judging the efficacy of a desensitization protocol. Clin Therap 37(6):1259–1269
Altwerger G, Gressel GM, English DP, Nelson WK, Carusillo N, Silasi D-A, Azodi M, Santin A, Schwartz PE, Ratner ES (2017) Platinum desensitization in patients with carboplatin hypersensitivity: a single-institution retrospective study. Gynecol Oncol 144(1):77–82
Robinson JB, Singh D, Bodurka-Bevers DC, Wharton JT, Gershenson DM, Wolf JK (2001) Hypersensitivity reactions and the utility of oral and intravenous desensitization in patients with gynecologic malignancies. Gynecol Oncol 82(3):550–558
Maindrault-Goebel F, André T, Tournigand C, Louvet C, Perez-Staub N, Zeghib N, De Gramont A (2005) Allergic-type reactions to oxaliplatin: retrospective analysis of 42 patients. Eur J Cancer 41(15):2262–2267
Lee S-Y, Kang H-R, Song W-J, Lee K-H, Han S-W, Cho SH (2014) Overcoming oxaliplatin hypersensitivity: different strategies are needed according to the severity and previous exposure. Cancer Chemother Pharmacol 73(5):1021–1029
Sohn K-H, Kang D-Y, Kim J-Y, Lee S-Y, Lee K-H, Han S-W, Kang H-R (2018) Incidence and risk of oxaliplatin-induced hypersensitivity in patients with asymptomatic prior exposure: a prospective observational study. J Allergy Clin Immunol Pract. https://doi.org/10.1016/j.jaip.2017.12.026
Weiss M, Hug MI, Neff T, Fischer J (2000) Syringe size and flow rate affect drug delivery from syringe pumps. Can J Anesth 47(10):1031–1035
Weiss M, Bänziger O, Neff T, Fanconi S (2000) Influence of infusion line compliance on drug delivery rate during acute line loop formation. Intensive Care Med 26(6):776–779
Alvarez-Cuesta E, Madrigal-Burgaleta R, Angel-Pereira D, Ureña-Tavera A, Zamora-Verduga M, Lopez-Gonzalez P, Berges-Gimeno M (2015) Delving into cornerstones of hypersensitivity to antineoplastic and biological agents: value of diagnostic tools prior to desensitization. Allergy 70(7):784–794
Wang AL, Patil SU, Long AA, Banerji A (2015) Risk-stratification protocol for carboplatin and oxaliplatin hypersensitivity: repeat skin testing to identify drug allergy. Ann Allergy Asthma Immunol 115(5):422–428
Patil SU, Long AA, Ling M, Wilson MT, Hesterberg P, Wong JT, Banerji A (2012) A protocol for risk stratification of patients with carboplatin-induced hypersensitivity reactions. J Allergy Clin Immunol 129(2):443–447
Leguy-Seguin V, Jolimoy G, Coudert B, Pernot C, Dalac S, Vabres P, Collet E (2007) Diagnostic and predictive value of skin testing in platinum salt hypersensitivity. J Allergy Clin Immunol 119(3):726–730
Zanotti K, Rybicki L, Kennedy A, Belinson J, Webster K, Kulp B, Peterson G, Markman M (2001) Carboplatin skin testing: a skin-testing protocol for predicting hypersensitivity to carboplatin chemotherapy. J Clin Oncol 19(12):3126–3129
Lee C-W, Matulonis UA, Castells MC (2004) Carboplatin hypersensitivity: a 6-h 12-step protocol effective in 35 desensitizations in patients with gynecological malignancies and mast cell/IgE-mediated reactions. Gynecol Oncol 95(2):370–376
Garufi C, Cristaudo A, Vanni B, Bria E, Aschelter A, Santucci B, Terzoli E (2003) Skin testing and hypersensitivity reactions to oxaliplatin. Ann Oncol 14(3):497–498
Markman M, Zanotti K, Kulp B, Peterson G, Markman M (2003) Relationship between a history of systemic allergic reactions and risk of subsequent carboplatin hypersensitivity. Gynecol Oncol 89(3):514–516
Bruchim I, Goldberg A, Fishman A, Confino-Cohen R (2014) Carboplatin hypersensitivity: evaluation and successful desensitization protocol. Immunotherapy 6(8):905–912
Acknowledgements
This research was supported by a Grant from Ministry of Food and Drug Safety to the regional pharmacovigilance center in 2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chung, S.J., Kang, SY., Kang, RY. et al. A new non-dilution rapid desensitization protocol successfully applied to all-grade platinum hypersensitivity. Cancer Chemother Pharmacol 82, 777–785 (2018). https://doi.org/10.1007/s00280-018-3662-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3662-0