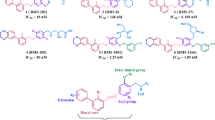
Abstract
Purpose
Poly(ADP-ribose) polymerase-1 (PARP-1) is a nuclear enzyme involved in the detection and repair of DNA damage. Studies have shown that inhibition of PARP and Tankyrase (TNKS) has significant antitumor effect in several types of cancers including BRCA-negative breast cancers.
Methods
Identification of ZYTP1, a novel PARP inhibitor, through a battery of in vitro assays and in vivo studies. PARP and TNKS inhibitory activity of ZYTP1 was assessed in cell-free kinase assay. In vitro cell killing potency of ZYTP1 was tested in a panel of cell lines including BRCA-negative cells. ZYTP1 was also tested in xenograft models in combination with temozolomide (TMZ). The pharmacokinetic profile of ZYTP1 was determined in rodent and non-rodent preclinical species. Safety of ZYTP1 was assessed in Wistar rats and Beagle dogs upon repeated dosing.
Results
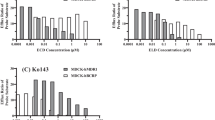
ZYTP1 inhibited PARP1, PARP2, Tankyrase-1 and Tankyrase-2 with IC50 of 5.4, 0.7, 133.3 and 289.8 nM, respectively, and additionally trapped PARP1 onto damaged DNA. It also potentiated MMS-mediated killing of different cancer cell lines. Compound demonstrated good Caco-2 cell permeability. The oral bioavailability of ZYTP1 in mice, rats and dogs ranged between 40 and 79% and demonstrated efficacy in colon cancer xenograft model at a dose of 1–10 mg/kg in combination with TMZ. In a 28-day repeat dosing, oral toxicity study in rats, it was found to show > 10× safety margin.
Conclusions
ZYTP1 is a novel PARP inhibitor that showed potential for development as a treatment for various solid tumors.







Similar content being viewed by others
References
Schreiber V, Dantzer F, Amé J-C, de Murcia G (2006) Poly(ADPribose): novel functions for an old molecule. Nat Rev Mol Cell Biol 7:517–528
Schiewer MJ, Knudsen KE (2014) Transcriptional roles of PARP1 in cancer. Mol Cancer Res 12:1069–1080
de Murcia JM, Niedergang C, Trucco C, Ricoul M, Dutrillaux B, Mark M, Oliver FJ, Masson M, Dierich A, LeMeur M, Walztinger C, Chambon P, de Murcia G (1997) Requirement of poly(ADP-ribose) polymerase in recovery from DNA damage in mice and in cells. Proc Natl Acad Sci USA 94:7303–7307
Bernstein C, Bernstein H, Payne CM, Garewal H (2002) DNA repair/pro-apoptotic dual-role proteins in five major DNA repair pathways: fail-safe protection against carcinogenesis. Mutat Res 511:145–178
Plummer E (2006) Inhibition of polyADP-ribose polymerase in cancer. Curr Opin Pharmacol 6:364–368
Liu X, Shi Y, Guan R, Donawho C, Luo Y, Palma J, Zhu GD, Johnson EF, Rodriguez LE, Ghoreishi-Haack N, Jarvis K, Hradil VP, Colon-Lopez M, Cox BF, Klinghofer V, Penning T, Rosenberg SH, Frost D, Giranda VL, Luo Y (2008) Potentiation of temozolomide cytotoxicity by poly(ADP)ribose polymerase inhibitor ABT-888 requires a conversion of single-stranded DNA damages to double-stranded DNA breaks. Mol Cancer Res 6:1621–1629
Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T (2005) Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434:913–917
Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, Martin NM, Jackson SP, Smith GC, Ashworth A (2005) Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434:917–921
McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, Swift S, Giavara S, O’Connor MJ, Tutt AN, Zdzienicka MZ, Smith GC, Ashworth A (2006) Deficiency in the repair of DNA damage by homologous recombination and sensitivity to polyADP-ribose polymerase inhibition. Cancer Res 66:8109–8115
Poggio F, Bruzzone M, Ceppi M, Conte B, Martel S, Maurer C, Tagliamento M, Viglietti G, Del Mastro L, de Azambuja E, Lambertini M (2018) Single-agent PARP inhibitors for the treatment of patients with BRCA-mutated HER2-negative metastatic breast cancer: a systematic review and meta-analysis. ESMO Open 20:e000361
Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H (1997) Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science 275:1784–1787
Nakashima T, Liu D, Nakano J, Ishikawa S, Yokomise H, Ueno M, Kadota K, Huang CL (2008) Wnt1 overexpression associated with tumor proliferation and a poor prognosis in non-small cell lung cancer patients. Oncol Rep 19:203–209
Nathubhai A, Haikarainen T, Koivunen J, Murthy S, Koumanov F, Lloyd MD, Holman GD, Pihlajaniemi T, Tosh D, Lehtiö L, Threadgill MD (2018) Highly potent and isoform selective dual site binding tankyrase/Wnt signaling inhibitors that increase cellular glucose uptake and have antiproliferative activity. J Med Chem 26:814–820
Riffell JL, Lord CJ, Ashworth A (2012) Tankyrase-targeted therapeutics: expanding opportunities in the PARP family. Nat Rev Drug Discov 11:923–936
Menear KA, Adcock C, Boulter R, Cockcroft X, Copsey L, Cranston A, Dillon KJ, Drzewiecki J, Garman S, Gomez S, Javaid H, Kerrigan F, Knights C, Lau A, Loh VM Jr, Matthews ITW, Moore S, O’Connor MJ, Smith GCM, Martin NMB (2008) 4-[3-(4-Cyclopropanecarbonylpiperazine-1-carbonyl)-4-fluorobenzyl]-2H-phthalazin-1-one: a novel bioavailable inhibitor of poly(ADP-ribose) polymerase-1. J Med Chem 51:6581–6591
Srivastava BK, Desai RC, Patel PR (2014) Substituted phthalazin-1 (2h)-one derivatives as selective inhibitors of poly(ADP-ribose) polymerase-1. WO 2014102817:A1
Karson SP, Paul JH (2004) An enzymatic assay for poly(ADP-ribose) polymerase-1 (PARP-1) via the chemical quantitation of NAD: application to the high-throughput screening of small molecules as potential inhibitors. Anal Biochem 326:78–86
Murai J, Huang SY, Das BB, Renaud A, Zhang Y, Doroshow JH, Ji J, Takeda S, Pommier Y (2012) Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res 72:5588–5599
Parker RM (2006) Testing for reproductive toxicity. In: Hood R (ed) Developmental and reproductive toxicology, a practical approach, 2nd edn. CRC Press, Boca Raton, pp 425–87
Maron DM, Ames BN (1983) Revised method for the Salmonella mutagenicity test. Mutat Res 113:173–215
Mortelmans K, Zeiger E (2000) The AMES Salmonella/microsome Mutagenicity assay. Mutation Res 455:29–60
Evans HJ, O’Riordan ML (1975) Human lymphocytes for analysis of chromosome aberrations in mutagen tests. Mutat Res 31:135–148
ICH Guideline (2012) Guidance on genotoxicity testing and data interpretation for pharmaceuticals intended for human use. S2 (R1)
OECD Guideline (1997) Bacterial reverse mutation test. No. 471
OECD Guideline (1997) In vitro mammalian chromosome aberration test. No. 473
Curtin NJ, Szabo C (2013) Therapeutic applications of PARP inhibitors: anticancer therapy and beyond. Mol Asp Med 34:1217–1256
Lupo B, Trusolino L (2014) Inhibition of poly(ADP-ribosyl) ation in cancer: old and new paradigms revisited. Biochem Biophys Acta 1846:201–215
Lord CJ, Ashworth A (2012) The DNA damage response and cancer therapy. Nature 481:287–294
Lopez-Serra L, Esteller M (2008) Proteins that bind methylated DNA and human cancer: reading the wrong words. Br J Cancer 98:1881–1885
Zhao H, Thienpont B, Yesilyurt BT, Moisse M, Reumers J, Coenegrachts L, Sagaert X, Schrauwen S, Smeets D, Matthijs G, Aerts S, Cools J, Metcalf A, Spurdle A, Amant ANECS, Lambrechts F D (2014) Mismatch repair deficiency endows tumors with a unique mutation signature and sensitivity to DNA double-strand break. Elife 3:e02725
Horton TM, Jenkins G, Pati D, Zhang L, Dolan ME, Ribes-Zamora A, Bertuch AA, Blaney SM, Delaney SL, Hegde M, Berg SL (2009) Poly(ADP-ribose) polymerase inhibitor ABT-888 potentiates the cytotoxic activity of temozolomide in leukemia cells: influence of mismatch repair status and O6-methylguanine-DNA methyltransferase activity. Mol Cancer Ther 8:2232–2242
Palma JP, Wang YC, Rodriguez LE, Montgomery D, Ellis PA, Bukofzer G, Niquette A, Liu X, Shi Y, Lasko L, Zhu GD, Penning TD, Giranda VL, Rosenberg SH, Frost DJ, Donawho CK (2009) ABT-888 confers broad in vivo activity in combination with temozolomide in diverse tumors. Clin Cancer Res 15:7277–7290
Jessica SB, Stan BK, Timothy AY (2016) PARP inhibitors: the race is on. Br J Cancer 114:713–715
McCabe N, Cerone MA, Ohishi T, Seimiya H, Lord CJ, Ashworth A (2009) Targeting tankyrase 1 as a therapeutic strategy for BRCA-associated cancer. Oncogene 28:1465–1470
Haikarainen T, Krauss S, Lehtio L (2014) Tankyrases: structure, function and therapeutic implications in cancer. Curr Pharm Des 20:6472–6488
McGonigle S, Chen Z, Wu J, Chang P, Kolber-Simonds D, Ackermann K, Twine NC, Shie JL, Miu JT, Huang KC, Moniz GA, Nomoto K (2015) E7449: a dual inhibitor of PARP1/2 and tankyrase1/2 inhibits growth of DNA repair deficient tumors and antagonizes Wnt signaling. Oncotarget 1:41307–41323
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors acknowledge that this work was supported by Zydus Research Centre, Ahmedabad, India and declare that there were no potential conflicts of interest.
Ethical approval
The current work involves the use of animals. All animal care and experimental procedures were in compliance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals as stated by the National Institutes of Health and were approved by the Institutional Animal Ethics Committee.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jain, M.R., Mohapatra, J., Bandhyopadhyay, D. et al. Identification and preclinical characterization of a novel and potent poly (ADP-ribose) polymerase (PARP) inhibitor ZYTP1. Cancer Chemother Pharmacol 82, 635–647 (2018). https://doi.org/10.1007/s00280-018-3653-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3653-1