Abstract
Purpose
We conducted a prospective study to evaluate the efficacy and safety of biweekly gemcitabine and carboplatin combination treatment in patients with resected non-small cell lung cancer (NSCLC).
Methods
Patients with completely resected stage IB to IIIA NSCLC were treated with four cycles of gemcitabine (1000 mg/m2, days 1 and 15) plus carboplatin [area under the time-concentration curve (AUC) 5 mg/mL/min, day 1] every 4 weeks as adjuvant chemotherapy.
Results
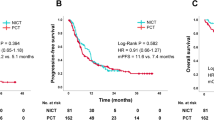
Forty-three patients were enrolled in this study. The median number of treatment cycles was four. The completion rate of chemotherapy was 79.1%. Major grade 3/4 hematological adverse events included leukocytopenia (27.9%) and neutropenia (53.5%), whereas non-hematological toxicities were generally mild. Ten patients (23.3%) required chemotherapy treatment schedule delay, and one patient required one dose level reduction because of drug fever. Median disease-free survival was 78.6 months [95% confidence interval (CI) 39.5–not reached (NA)] and median overall survival was not reached (95% CI 83.7–NA).
Conclusions
Biweekly administration of gemcitabine and carboplatin is effective and well tolerated for patients with completely resected NSCLC as an adjuvant chemotherapy.


Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A et al (2016) Cancer statistics, 2016. CA Cancer J Clin 66:7–30
Peters S, Weder W, Dafni U et al (2014) Lungscape: resected non-small-cell lung cancer outcome by clinical and pathological parameters. J Thorac Oncol 9:1675–1684
Buffoni L, Vavalà T, Novello S (2016) Adjuvant therapy of resected non-small cell lung cancer: can we move forward? Curr Treat Options Oncol 17:54
Pignon JP, Tribodet H, Scagliotti GV et al (2008) Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE collaborative group. J Clin Oncol 26:3552–3559
Douillard JY, Tribodet H, Aubert D et al (2010) Adjuvant cisplatin and vinorelbine for completely resected non-small cell lung cancer. J Thorac Oncol 5:220–228
Okumura N, Sonobe M, Okabe K et al (2017) Feasibility of adjuvant chemotherapy with S-1 plus carboplatin followed by single-agent maintenance therapy with S-1 for completely resected non-small-cell lung cancer: results of the setouchi lung cancer group study 1001. Int J Clin Oncol 22:274–282
Schmid-Bindert G, Engel-Riedel W, Reck M et al (2015) A randomized phase 2 study of pemetrexed in combination with cisplatin or carboplatin as adjuvant chemotherapy in patinets with completely resected stage IB or II non-small-cell lung cancer. Lung Cancer 90:397–404
Barton-Burke M (1999) Gemcitabine: a pharmacologic and clinical overview. Cancer Nurs 22:176–183
Ohe Y, Ohashi Y, Kubota K et al (2007) Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: Four-arm cooperative study in Japan. Ann Oncol 18:317–323
Scagliotti GV, Parikh P, von Pawel J et al (2008) Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 26:3543–3551
Zatloukal P, Petruzelka L, Zemanov M et al (2003) Gemcitabine plus cisplatin vs. gemcitabine plus carboplatin in stage IIIb and IV non-small cell lung cancer: a phase III randomized trial. Lung Cancer 41:321–331
Tomizawa Y, Ishihara S, Iijima H et al (2007) A phase I dose escalation study of biweekly gemcitbine and carbopatin in completely resected stage IB-IIIA nonsmall cell lung cancer. Am J Clin Oncol 30:498–502
Jiang J, Liang X, Zhou X et al (2007) A meta-analysis of randomized controlled trials comparing carboplatin-based to cisplatin-based chemotherapy in advanced non-small cell lung cancer. Lung Cancer 57:348–358
Ardizzoni A, Boni L, Tiseo M, CISCA (CISplatin versus CArboplatin) Meta-analysis Group et al (2007) Cisplatin versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: an individual patient data meta-analysis. J Natl Cancer Inst 99:847–857
Hotta K, Matsuo K, Ueoka H et al (2004) Meta-analysis of randomized clinical trials comparing cisplatin to carboplatin in patients with advanced non-small-cell lung cancer. J Clin Oncol 22:3852–3859
Abratt RP, Bezwoda WR, Falkson G et al (1994) Efficacy and safety profile of gemcitabine in non-small-cell lung cancer: a phase II study. J Clin Oncol 12:1535–1540
Anderson H, Lund B, Bach F et al (1994) Single-agent activity of weekly gemcitabine in advanced non-small-cell lung cancer: a phase II study. J Clin Oncol 12:1821–1826
Gatzemeier U, Shepherd FA, Le Chevalier T et al (1996) Activity of gemcitabine in patients with non-small cell lung cancer: a multicenter, extended phase II study. Eur J Cancer 32A:243–248
Winton T, Livingston R, Johnson D et al (2005) Vinorelbine plus cisplatin vs observation in resected non-small-cell lung cancer. N Engl J Med 352:2589–2597
Douillard J, Rosell R, De Lena M et al (2006) Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer [Adjuvant Navelbine International Trialist Association (ANITA)]: a randomized controlled trial. Lancet Oncol 7:719–727
Lopez-Vivanco G, Viteri A, Barcelo R et al (2005) Biweekly administration of cisplatin/gemcitabine in advanced nonsmall cell lung cancer. Am J Clin Oncol 28:501–507
Uramoto H, Nakanishi R, Nagashima A et al (2010) A randomized phase II trial of adjuvant chemotherapy with bi-weekly carboplatin plus paclitaxel versus carboplatin plus gemcitabine in patients with completely resected non-small cell lung cancer. Anticancer Res 30:4695–4700
Usami N, Yokoi K, Hasegawa Y et al (2010) Phase II study of carboplatin and gemcitabine as adjuvant chemotherapy in patients with completely resected non-small cell lung cancer: a report from the Central Japan Lung Study Group, CJLSG 0503 trial. Int J Clin Oncol 15:583–587
Acknowledgements
We thank Dr. Kuniaki Suzuki and Dr. Yosuke Kamide for their help with the data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author Reiko Sakurai declares that she has no conflict of interest. Author Yoshio Tomizawa declares that he has no conflict of interest. Author Akihiro Yoshii declares that he has no conflict of interest. Author Yosuke Miura declares that he has no conflict of interest. Author Hiroaki Tsurumaki declares that he has no conflict of interest. Author Kyoichi Kaira declares that he has no conflict of interest. Author Noriaki Sunaga declares that he has no conflict of interest. Author Osamu Kawashima declares that he has no conflict of interest. Author Takeshi Hisada declares that he has no conflict of interest. Author Masanobu Yamada declares that he has no conflict of interest. Author Ryusei Saito declares that he has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Yoshio Tomizawa—Deceased.
Rights and permissions
About this article
Cite this article
Sakurai, R., Tomizawa, Y., Yoshii, A. et al. A phase II study of biweekly gemcitabine and carboplatin in completely resected stage IB-IIIA non-small cell lung cancer. Cancer Chemother Pharmacol 81, 103–109 (2018). https://doi.org/10.1007/s00280-017-3439-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3439-x