Abstract
Purpose
RO5323441 is a humanized anti-placental growth factor (PlGF) monoclonal antibody that has shown preclinical activity in several cancer models. The objective of this analysis is to examine the pharmacokinetic (PK) results from four Phase I studies that have been conducted with RO5323441 (n = 61) and to report an apparent drug–drug interaction observed when RO5323441 was administered in combination with bevacizumab.
Methods
The four Phase I studies were a multiple-ascending dose study in 23 patients with solid tumors (Study 1), an open-label study in seven patients with colorectal/ovarian cancer (Study 2), a sorafenib combination study in nine patients with hepatocellular carcinoma (Study 3), and a bevacizumab combination study in 22 patients with recurrent glioblastoma (Study 4). A two-compartment linear population PK model was developed from these four studies to characterize the PK of RO5323441 in patients with cancer.
Results
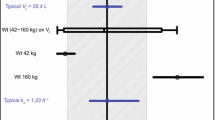
The PK properties of RO5323441 were similar in the first three studies. However, substantially higher RO5323441 exposures were observed in Study 4 when RO5323441 was administered in combination with bevacizumab. A linear two-compartmental population PK model indicated that the co-administration of bevacizumab would decrease the clearance of RO5323441 by 53%. Clinical data suggested that the decrease in RO5323441 clearance was inversely associated with bevacizumab exposure.
Conclusions
The exact reason for the increase in RO5323441 exposure following bevacizumab co-administration is not currently known. One possibility is a drug–drug interaction via a target-trapping mechanism that is mediated by the vascular endothelial growth factor receptor-1 (VEGFR-1).





Similar content being viewed by others
References
Folkman J (2007) Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov 6:273–286
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Oklu R, Walker TG, Wicky S, Hesketh R (2010) Angiogenesis and current antiangiogenic strategies for the treatment of cancer. J Vasc Interv Radiol 21:1791–1805
Cook KM, Figg WD (2010) Angiogenesis inhibitors: current strategies and future prospects. CA Cancer J Clin 60:222–243
Chen C-N, Hsieh F-J, Cheng Y-M et al (2004) The significance of placenta growth factor in angiogenesis and clinical outcome of human gastric cancer. Cancer Lett 213:73–82
Yang W, Ahn H, Hinrichs M et al (2003) Evidence of a novel isoform of placenta growth factor (PlGF-4) expressed in human trophoblast and endothelial cells. J Reprod Immunol 60:53–60
Cai J, Ahmad S, Jiang WG et al (2003) Activation of vascular endothelial growth factor receptor-1 sustains angiogenesis and Bcl-2 expression via the phosphatidylinositol 3-kinase pathway in endothelial cells. Diabetes 52:2959–2968
Autiero M, Luttun A, Tjwa M, Carmeliet P (2003) Placental growth factor and its receptor, vascular endothelial growth factor receptor-1: novel targets for stimulation of ischemic tissue revascularization and inhibition of angiogenic and inflammatory disorders. J Thromb Haemost 1:1356–1370
Xu H-X, Zhu X-D, Zhuang P-Y et al (2011) Expression and prognostic significance of placental growth factor in hepatocellular carcinoma and peritumoral liver tissue. Int J Cancer 128:1559–1569
Escudero-Esparza A, Martin TA, Davies ML, Jiang WG (2009) PGF isoforms, PLGF-1 and PGF-2, in colorectal cancer and the prognostic significance. Cancer Genom Proteom 6:239–246
Pompeo E, Albonici L, Doldo E et al (2009) Placenta growth factor expression has prognostic value in malignant pleural mesothelioma. Ann Thorac Surg 88:426–431
Cheng S-J, Lee J-J, Kok S-H et al (2010) Expression of placenta growth factor: an independent factor for prediction of progression and prognosis of oral cancer. Head Neck 32:1363–1369
Sung CY, Son MW, Ahn TS et al (2012) Expression of placenta growth factor in colorectal carcinomas. J Korean Soc Coloproctol 28:315–320
Snuderl M, Batista A, Kirkpatrick ND et al (2013) Targeting placental growth factor/neuropilin 1 pathway inhibits growth and spread of medulloblastoma. Cell 152:1065–1076
Lassen U, Nielsen DL, Sørensen M et al (2012) A phase I, dose-escalation study of TB-403, a monoclonal antibody directed against PlGF, in patients with advanced solid tumours. Br J Cancer 106:678–684
Fischer C, Jonckx B, Mazzone M et al (2007) Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell 131:463–475
Martinsson-Niskanen T, Riisbro R, Larsson L et al (2011) Monoclonal antibody TB-403: a first-in-human, Phase I, double-blind, dose escalation study directed against placental growth factor in healthy male subjects. Clin Ther 33:1142–1149
Lassen U, Chinot OL, McBain C, et al (2015) Phase 1 dose-escalation study of the antiplacental growth factor monoclonal antibody RO5323441 combined with bevacizumab in patients with recurrent glioblastoma. Neuro-Oncol 17:1007–1015
Lu J-F, Bruno R, Eppler S et al (2008) Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother Pharmacol 62:779–786
Gabrielsson J, Weiner D (2000) Pharmacokinetic and Pharmacodynamic Data Analysis: Concepts & Application, 3rd Ed, Swedish Pharmaceutical Press, Stockholm, Sweden
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO (2011) Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J 13(2):143–151. doi:10.1208/s12248-011-9255-z
Ferrara N, Gerber H-P, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9:669–676
Keizer RJ, Huitema ADR, Schellens JHM, Beijnen JH (2010) Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet 49:493–507
Wang W, Wang EQ, Balthasar JP (2008) Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther 84:548–558
Czock D, Keller F, Seidling HM (2012) Pharmacokinetic predictions for patients with renal impairment: focus on peptides and protein drugs. Br J Clin Pharmacol 74:66–74
Han K, Jin J, Maia M et al (2014) Lower exposure and faster clearance of bevacizumab in gastric cancer and the impact of patient variables: analysis of individual data from AVAGAST Phase III trial. AAPS J 16:1056–1063
Shibuya M (2011) Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis a crucial target for anti- and pro-angiogenic therapies. Genes Cancer 2:1097–1105
Li B, Wang C, Zhang Y et al (2013) Elevated PLGF contributes to small-cell lung cancer brain metastasis. Oncogene 32:2952–2962
Coenegrachts L, Schrauwen S, Van Bree R et al (2012) Increased expression of placental growth factor in high-grade endometrial carcinoma. Oncol Rep 29:413–418
Kopparapu PK, Boorjian SA, Robinson BD et al (2013) Expression of VEGF and its receptors VEGFR1/VEGFR2 is associated with invasiveness of bladder cancer. Anticancer Res 33:2381–2390
Acknowledgements
The authors would like to thank the patients and their families for their participation in this study, and the staff at the study sites, in particular the Department of Neurology, University Hospital of Zürich, Zürich, Switzerland; Department of Oncology, Rigshospitalet, Copenhagen, Denmark; Division of Medical Oncology, National Cancer Center, Singapore; National University Cancer Institute, Singapore; Neuro-oncologie, Hôpital de la Timone, Marseille, France; Clinical Oncology, Christie Hospital NHS Trust, Manchester, United Kingdom. Support for third-party writing assistance for this article, furnished by Mike Parsons, PhD and Jamie Ashman, PhD, was provided by Prism Ideas.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All of the authors are employees of Roche.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the studies.
Rights and permissions
About this article
Cite this article
Wang, K., Stark, F.S., Schlothauer, T. et al. An apparent clinical pharmacokinetic drug–drug interaction between bevacizumab and the anti-placental growth factor monoclonal antibody RO5323441 via a target-trapping mechanism. Cancer Chemother Pharmacol 79, 661–671 (2017). https://doi.org/10.1007/s00280-017-3242-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3242-8