Abstract
Purpose
Acid-suppression therapy is known to decrease the systemic exposure of erlotinib. The erlotinib prescribing information recommends staggering dosing with a histamine-2 receptor antagonist (H2RA) and avoiding concurrent use of a proton pump inhibitor (PPI). This retrospective analysis evaluated the frequency of concurrent acid-suppression therapy in oncology patients receiving erlotinib and its association with outcomes.
Methods
All patients prescribed erlotinib within UC San Diego Health System between February 26, 2011, and February 28, 2014, were assessed for eligibility, survival outcomes and adverse events.
Results
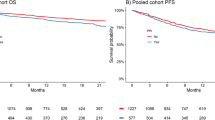
Of the 76 patients in the analysis, 24 were prescribed both a PPI and an H2RA with erlotinib therapy (31.6 %). The two patient groups, with (n = 24) and without PPI/H2RA (n = 52), were similar in clinical characteristics and erlotinib dose. One patient received an H2RA therapy alone and was excluded from the analysis; no one received PPI therapy alone. Patients receiving erlotinib alone had a longer median progression-free survival (PFS) compared to patients with concurrent PPI/H2RA therapy (11.0 months vs. 5.3 months; P = 0.029). Overall survival (OS) and incidence of rash and/or diarrhea did not correlate with use of acid-suppression therapy.
Conclusion
Nearly one-third of patients received acid-suppression therapy. Patients treated with erlotinib and PPI/H2RA therapy had shorter PFS, but similar OS and adverse event profile compared to those who did not receive acid-suppression.


Similar content being viewed by others
References
Borad MJ, Curtis KK, Babiker HM et al (2012) The impact of concomitant medication use on patient eligibility for phase I cancer clinical trials. J Cancer 3:345–353
Smelick GS, Heffron TP, Chu L et al (2013) Prevalence of acid-reducing agents (ARA) in cancer populations and ARA drug-drug interaction potential for molecular targeted agents in clinical development. Mol Pharm 10(11):4055–4062
Bosulif (bosutinib) [prescribing information]. New York, NY: Pfizer, Inc; 2016. Accessed April 19, 2016. http://labeling.pfizer.com/ShowLabeling.aspx?id=884
Sprycel (dasatinib) [prescribing information]. Princeton, NJ: Bristol-Myers Squibb Company; 2015. Accessed 19 April, 2016. http://packageinserts.bms.com/pi/pi_sprycel.pdf
Ibrance (palbociclib) [prescribing information]. New York, NY: Pfizer, Inc; 2016. http://labeling.pfizer.com/ShowLabeling.aspx?id=2191. Accessed 19 April, 2016
Tarceva (erlotinib) [prescribing information]. Northbrook, IL: OSI Pharmaceuticals, LLC: 2015. Accessed 19 April, 2016. http://www.gene.com/download/pdf/tarceva_prescribing.pdf
Kletzl H, Giraudon M, Ducray PS et al (2015) Effect of gastric pH on erlotinib pharmacokinetics in healthy individuals: omeprazole and ranitidine. Anticancer Drugs 26(5):565–572
Chu MP, Ghosh S, Chambers CR et al (2015) Gastric Acid suppression is associated with decreased erlotinib efficacy in non-small-cell lung cancer. Clin Lung Cancer 16(1):33–39
Hilton JF, Tu D, Seymour L et al (2013) An evaluation of the possible interaction of gastric acid suppressing medication and the EGFR tyrosine kinase inhibitor erlotinib. Lung Cancer 82(1):136–142
University of California, San Diego. Study of Personalized Cancer Therapy to Determine Response and Toxicity (UCSD_PREDICT). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [cited 2016 April 21]. https://clinicaltrials.gov/show/NCT02478931. NLM Identifier: NCT02478931
Therneau T. A Package for Survival Analysis in S. version 2.38. 2015. http://CRAN.R-project.org/package=survival
R Core Team.(2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer. Version 4.2016. Accessed April 21, 2016. http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
National Comprehensive Cancer Network. NCCN Clinical practice guidelines in oncology: Pancreatic Adenocarcinoma. Version 1.2016. Accessed April 21, 2016. http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf
DeVault KR, Castell DO (2005) American College of G. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol 100(1):190–200
Rosell R, Carcereny E, Gervais R et al (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13(3):239–246
Acknowledgments
The authors would like to thank Andrew Chang and Sariah Liu for their assistance with study start-up.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Lisa H. Lam is a postdoctoral fellow with funding supported by Pfizer Global Research and Development and the Skaggs School of Pharmacy and Pharmaceutical Sciences at the University of California, San Diego. Edmund V. Capparelli has consultant funds from Gilead Sciences, Alexion Pharmaceuticals, The Medicines Company, and Cempra. Razelle Kurzrock has research funding from Guardant, Sequenom, Foundation Medicine, Merck Serono, Pfizer, and Genentech, consultant funds from Sequenom, Actuate Therapeutics and X-Biotech, and an ownership interest in CureMatch Inc. and Novena Inc.
Rights and permissions
About this article
Cite this article
Lam, L.H., Capparelli, E.V. & Kurzrock, R. Association of concurrent acid-suppression therapy with survival outcomes and adverse event incidence in oncology patients receiving erlotinib. Cancer Chemother Pharmacol 78, 427–432 (2016). https://doi.org/10.1007/s00280-016-3087-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3087-6