Abstract
Purpose
Inhibition of transforming growth factor-beta receptor I (TGF-beta RI)-mediated signaling pathways blocks tumor growth and metastases in nonclinical studies. Galunisertib (LY2157299), a small molecule inhibitor of TGF-beta RI serine/threonine kinase, had antitumor effects with acceptable safety/tolerability in a first-in-human dose (FHD) study conducted mainly in Caucasian patients with glioma. In this nonrandomized, open-label, dose-escalation study, we assessed safety/tolerability, pharmacokinetics (PK), and tumor response in Japanese patients.
Methods
Patients with advanced and/or metastatic disease refractory were assigned sequentially to Cohort-1 (80 mg) or Cohort-2 (150 mg) of galunisertib, administered twice daily and treated using 2-week on, 2-week off treatment cycles. Dose escalation was guided by predefined PK criteria and dose-limiting toxicities (DLT). Safety assessments included treatment-emergent adverse events (TEAEs) and cardiac safety (ultrasound cardiography/Doppler imaging, electrocardiogram, chest computed tomography, and cardiotoxicity serum biomarkers).
Results
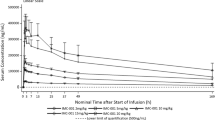
Twelve patients (Cohort-1, n = 3; Cohort-2, n = 9) were enrolled and the most common types of cancer were pancreatic (n = 5) and lung cancer (n = 3). Seven patients (Cohort-1, n = 2; Cohort-2, n = 5) experienced possibly galunisertib-related TEAEs. The most frequent related TEAEs were brain natriuretic peptide increased (n = 2), leukopenia (n = 2), and rash (n = 2). No cardiovascular toxicities or other DLTs were reported. PK profile of galunisertib was consistent with the FHD study. Maximum plasma concentration was reached within 2 h post-dose, and the mean elimination half-life was 9 h.
Conclusions
Galunisertib had an acceptable tolerability and safety profile in Japanese patients with advanced cancers.
ClinicaTrials.gov. Identifier
NCT01722825.


Similar content being viewed by others
References
Massague J, Blain SW, Lo RS (2000) TGFbeta signaling in growth control, cancer, and heritable disorders. Cell 103:295–309. doi:10.1016/S0092-8674(00)00121-5
Pasche B (2001) Role of transforming growth factor beta in cancer. J Cell Physiol 186:153–168. doi:10.1002/1097-4652(200002)186:2<153:AID-JCP1016>3.0.CO;2-J
Akhurst RJ, Hata A (2012) Targeting the TGFbeta signalling pathway in disease. Nat Rev Drug Discov 11:790–811. doi:10.1038/nrd3810
Massague J (1998) TGF-beta signal transduction. Annu Rev Biochem 67:753–791. doi:10.1146/annurev.biochem.67.1.753
Roberts AB, Anzano MA, Lamb LC, Smith JM, Sporn MB (1981) New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci USA 78:5339–5343
Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl JH (1986) Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA 83:4167–4171
Zavadil J, Bottinger EP (2005) TGF-beta and epithelial-to-mesenchymal transitions. Oncogene 24:5764–5774. doi:10.1038/sj.onc.1208927
Bhola NE, Balko JM, Dugger TC, Kuba MG, Sanchez V, Sanders M, Stanford J, Cook RS, Arteaga CL (2013) TGF-beta inhibition enhances chemotherapy action against triple-negative breast cancer. J Clin Investig 123:1348–1358. doi:10.1172/JCI65416
Bueno L, de Alwis DP, Pitou C, Yingling J, Lahn M, Glatt S, Trocóniz IF (2008) Semi-mechanistic modelling of the tumour growth inhibitory effects of LY2157299, a new type I receptor TGF-beta kinase antagonist, in mice. Eur J Cancer 44:142–150. doi:10.1016/j.ejca.2007.10.008
Dituri F, Mazzocca A, Peidro FJ, Papappicco P, Fabregat I, De Santis F, Paradiso A, Sabbà C, Giannelli G (2013) Differential inhibition of the TGF-beta signaling pathway in HCC cells using the small molecule inhibitor LY2157299 and the D10 monoclonal antibody against TGF-beta receptor type II. PLoS ONE 8:e67109. doi:10.1371/journal.pone.0067109
Giannelli G, Villa E, Lahn M (2014) Transforming growth factor-beta as a therapeutic target in hepatocellular carcinoma. Cancer Res 74:1890–1894. doi:10.1158/0008-5472.CAN-14-0243
Lahn M, Kloeker S, Berry BS (2005) TGF-beta inhibitors for the treatment of cancer. Expert Opin Investig Drugs 14:629–643. doi:10.1517/13543784.14.6.629
Maier A, Peille AL, Vuaroqueaux V, Lahn M (2015) Anti-tumor activity of the TGF-beta receptor kinase inhibitor galunisertib (LY2157299 monohydrate) in patient-derived tumor xenografts. Cell Oncol 38:131–144. doi:10.1007/s13402-014-0210-8
Leivonen SK, Kahari VM (2007) Transforming growth factor-beta signaling in cancer invasion and metastasis. Int J Cancer 121:2119–2124. doi:10.1002/ijc.23113
Sawyer JS, Anderson BD, Beight DW, Campbell RM, Jones ML, Herron DK, Lampe JW, McCowan JR, McMillen WT, Mort N, Parsons S, Smith EC, Vieth M, Weir LC, Yan L, Zhang F, Yingling JM (2003) Synthesis and activity of new aryl- and heteroaryl-substituted pyrazole inhibitors of the transforming growth factor-beta type I receptor kinase domain. J Med Chem 46:3953–3956. doi:10.1021/jm0205705
Rodon J, Carducci MA, Sepulveda-Sanchez JM, Azaro A, Calvo E, Seoane J, Braña I, Sicart E, Gueorguieva I, Cleverly AL, Pillay NS, Desaiah D, Estrem ST, Paz-Ares L, Holdhoff M, Blakeley J, Lahn MM, Baselga J (2014) First-in-human dose study of the novel transforming growth factor-beta receptor I kinase inhibitor LY2157299 monohydrate in patients with advanced cancer and glioma. Clin Cancer Res. doi:10.1158/1078-0432.CCR-14-1380
Faivre SJ, Santoro A, Kelley RK, Merle P, Gane E, Douillard J-Y, Waldschmidt D, Mulcahy MF, Costentin C, Minguez B, Papappicco P, Gueorguieva I, Cleverly A, Desaiah D, Lahn MM, Ameryckx S, Benhadji KA, Raymond E, Giannelli G (2014) A phase 2 study of a novel transforming growth factor-beta (TGF-β1) receptor I kinase inhibitor, LY2157299 monohydrate (LY), in patients with advanced hepatocellular carcinoma (HCC). ASCO Gastrointestinal Cancers Symposium: J Clin Oncol 32:suppl 3; abstract LBA173
Anderton MJ, Mellor HR, Bell A, Sadler C, Pass M, Powell S, Steele SJ, Roberts RR, Heier A (2011) Induction of heart valve lesions by small-molecule ALK5 inhibitors. Toxicol Pathol 39:916–924. doi:10.1177/0192623311416259
First in human dose escalation study of TEW-7197 in subjects with advanced stage solid tumors. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02160106?term=TEW7197&rank=1. Accessed 22 March 2015
Stauber AJ, Credille KM, Truex LL, Ehlhardt WJ, Young JK (2014) Nonclinical safety evaluation of a transforming growth factor b receptor I kinase inhibitor in Fischer 344 rats and beagle dogs. J Clin Toxicol. doi:10.4172/2161-0495.196
Kovacs RJ, Maldonado G, Azaro A, Fernandez MS, Romero FL, Sepulveda-Sanchez JM, Corretti M, Carducci M, Dolan M, Gueorguieva I, Cleverly AL, Pillay NS, Baselga J, Lahn MM (2014) Cardiac safety of TGF-beta receptor I kinase inhibitor LY2157299 monohydrate in cancer patients in a first-in-human dose study. Cardiovasc Toxicol. doi:10.1007/s12012-014-9297-4
Gueorguieva I, Cleverly AL, Stauber A, Sada Pillay N, Rodon JA, Miles CP, Yingling JM, Lahn MM (2014) Defining a therapeutic window for the novel TGF-beta inhibitor LY2157299 monohydrate based on a pharmacokinetic/pharmacodynamic model. Br J Clin Pharmacol 77:796–807. doi:10.1111/bcp.12256
Kozloff M, Carbonero R, Nadal T, Gueorguieva I, Cleverly A, Desaiah D, Lahn MMF, Pillay S, Blunt A, Josep Tabernero, Macarulla T (2013) Phase Ib study evaluating safety and pharmacokinetics (PK) of the oral transforming growth factor-beta (TGF-ss) receptor I kinase inhibitor LY2157299 monohydrate (LY) when combined with gemcitabine in patients with advanced cancer. ASCO Meet Abstr 31:2563
Suarez C, Rodon J, Desjardins A, Forsyth PAJ, Gueorguieva I, Cleverly A, Burkholder T, Desaiah D, Lahn MMF, Wick W (2013) Phase Ib study evaluating safety and pharmacokinetics (PK) of the oral transforming growth factor-beta (TGF-ss) receptor I kinase inhibitor LY2157299 monohydrate (LY) when combined with chemoradiotherapy in newly diagnosed malignant gliomas. ASCO Meet Abst 31:2039
A study of LY2157299 in participants with myelodysplastic syndromes. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/results/NCT02008318. Accessed 26 Feb 2015
Rodon J, Carducci M, Sepulveda-Sanchez JM, Azaro A, Calvo E, Seoane J, Brana I, Sicart E, Gueorguieva I, Cleverly A, Pillay NS, Desaiah D, Estrem ST, Paz-Ares L, Holdhoff M, Blakeley J, Lahn MM, Baselga J (2015) Pharmacokinetic, pharmacodynamic and biomarker evaluation of transforming growth factor-beta receptor I kinase inhibitor, galunisertib, in phase 1 study in patients with advanced cancer. Investig New Drugs 33:357–370. doi:10.1007/s10637-014-0192-4
Acknowledgments
We thank all the patients who participated in this study, their families, and all the study site staff who assisted with this study.
Funding
This study was sponsored by Eli Lilly and Company. Medical writing assistance was provided by Julie A. Ely, PhD, CMPP, and Serina Stretton, PhD, CMPP, of ProScribe—Envision Pharma Group, and was funded by Eli Lilly Japan K.K.
Role of the sponsor
Eli Lilly and Company provided study drug (galunisertib) and was also involved in the study design, data collection, data analysis, and preparation of the manuscript.
Role of contributors
All authors participated in the interpretation of study results and in the drafting, critical revision, and approval of the final version of the manuscript. HA, OT, IG, and TT were involved in the study design and HA, OT, KO, IG, and TT were involved in the data analyses. YF, NY, HN, KS, YY, HU, and TT were investigators in the study. All authors had full access to the data in the study and the final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and animal rights
All procedures performed in this study involving human participants were in accordance with the ethical standards of the Institutional Review Board and with the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Fujiwara, Y., Nokihara, H., Yamada, Y. et al. Phase 1 study of galunisertib, a TGF-beta receptor I kinase inhibitor, in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol 76, 1143–1152 (2015). https://doi.org/10.1007/s00280-015-2895-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2895-4