Abstract
Purpose
Aggressive chemotherapy protocols for non-metastatic limb osteosarcoma have improved histological response without affecting prognosis. This study evaluated the toxicity and outcome of a dose-intensive, high-dose 3- to 5-drug pilot protocol, SCOS 89.
Methods
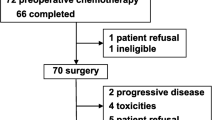
The cohort included 26 patients (14 male; ages 6.5–22 years) with non-metastatic limb osteosarcoma treated at a tertiary pediatric medical center between 1989 and 2013. Preoperatively, patients received two courses of once-weekly pulses of high-dose methotrexate (12–30 g/m2) for 2 weeks; doxorubicin (90 mg/m2) with dexrazoxane, combined with cisplatin (200 mg/m2), was added in week 3. Following methotrexate, 760 mg/m2 of folinic acid was administered. Postoperative chemotherapy was continued to a total of 14 courses of methotrexate, doxorubicin (up to a total dose of 360 mg/m2), and cisplatin (up to a total dose of 560 mg/m2). If toxicity occurred or <90 % tumor necrosis, ifosfamide (12 g/m2) plus etoposide (500 mg/m2) was substituted for doxorubicin, cisplatin, or methotrexate. Toxicity and death rates were calculated.
Results
All patients underwent definitive limb salvage surgery. Six patients died of infection, recurrent disease, or secondary malignancy. Median follow-up was 100 months (range 2–290). Event-free and overall survival rates, respectively, were 88 and 96 % at 2 years, 80 and 87.6 % at 5 years, 80 and 78 % at 10 years. Eleven patients required ifosfamide/etoposide substitution. One patient had a transient decreased left ventricular ejection fraction. Two patients developed acute nephrotoxicity during therapy, but no neurotoxicity. Seven patients had hearing impairment.
Conclusions
The SCOS 89 yields a high event-free survival rate with reduced nephro-/neuro-/cardiotoxicity in patients with non-metastatic limb osteosarcoma.

Similar content being viewed by others
References
ESMO/European Sarcoma Network Working Group (2012) Bone sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 23:vii100–vii109
Marina N, Gebhardt M, Teot L, Gorlick R (2004) Biology and therapeutic advances for pediatric osteosarcoma. Oncologist 9:422–441
Cortes EP, Holland JF, Wang JJ, Sinks LF (1972) Doxorubicin in disseminated osteosarcoma. JAMA 221:1132–1138
Jaffe N, Frei E 3rd, Traggis D, Bishop Y (1974) Adjuvant methotrexate and citrovorum-factor treatment of osteogenic sarcoma. N Engl J Med 291:994–997
Baum ES, Gaynon P, Greenberg L, Krivit W, Hammond D (1979) Phase II study of cis-dichlorodiammineplatinum(II) in childhood osteosarcoma: Children’s Cancer Study Group Report. Cancer Treat Rep 63:1621–1627
Ferrari S, Smeland S, Mercuri M, Italian and Scandinavian Sarcoma Groups et al (2005) Neoadjuvant chemotherapy with high-dose ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol 23:8845–8852
Bielack SS, Kempf-Bielack B, Delling G et al (2002) Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol 20:776–790
Bramwell VH, Burgers M, Sneath R et al (1992) A comparison of two short intensive adjuvant chemotherapy regimens in operable osteosarcoma of limbs in children and young adults: the first study of the European Osteosarcoma Intergroup. J Clin Oncol 10:1579–1591
Souhami RL, Craft AW, Van der Eijken JW et al (1997) Randomised trial of two regimens of chemotherapy in operable osteosarcoma: a study of the European Osteosarcoma Intergroup. Lancet 350:911–917
Provisor AJ, Ettinger LJ, Nachman JB et al (1997) Treatment of nonmetastatic osteosarcoma of the extremity with preoperative and postoperative chemotherapy: a report from the Children’s Cancer Group. J Clin Oncol 15:76–84
Fuchs N, Bielack SS, Epler D et al (1998) Long-term results of the co-operative German–Austrian–Swiss osteosarcoma study group’s protocol COSS-86 of intensive multidrug chemotherapy and surgery for osteosarcoma of the limbs. Ann Oncol 9:893–899
Ferrari S, Mercuri M, Picci P et al (1999) Nonmetastatic osteosarcoma of the extremity: results of a neoadjuvant chemotherapy protocol (IOR/OS-3) with high-dose methotrexate, intraarterial or intravenous cisplatin, doxorubicin, and salvage chemotherapy based on histologic tumor response. Tumori 85:458–464
Bacci G, Ferrari S, Bertoni F et al (2000) Long-term outcome for patients with nonmetastatic osteosarcoma of the extremity treated at the istituto ortopedico rizzoli according to the istituto ortopedico rizzoli/osteosarcoma-2 protocol: an updated report. J Clin Oncol 18:4016–4027
Lewis IJ, Nooij MA, Whelan J, MRC BO06 and EORTC 80931 collaborators, European Osteosarcoma Intergroup et al (2007) Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy: a randomized phase III trial of the European Osteosarcoma Intergroup. J Natl Cancer Inst 99:112–128
McTiernan A, Jinks RC, Sydes MR et al (2012) Presence of chemotherapy-induced toxicity predicts improved survival in patients with localized extremity osteosarcoma treated with doxorubicin and cisplatinum: a report from the European osteosarcoma study group. Eur J Cancer 48:703–712
Saeter G, Alvegård TA, Elomaa I, Stenwig AE et al (1991) Treatment of osteosarcoma of the extremities with the T-10 protocol, with emphasis on the effects of preoperative chemotherapy with single-agent high-dose methotrexate: a Scandinavian Sarcoma Group study. J Clin Oncol 9(10):1766–1775
Goldie JH, Coldman AJ (1979) A mathematic model for relating the drug sensitivity of tumors to their spontaneous mutation rate. Cancer Treat Rep 63:1727–1733
Bonadonna G, Valagussa P (1981) Dose response effect of adjuvant chemotherapy in breast-cancer. New Engl J Med 304:10–15
De Vita VT (1986) Dose-response is alive and well. J Clin Oncol 4:1157–1159
Winkler K, Beron G, Delling G et al (1988) Neoadjuvant chemotherapy of osteosarcoma: results of a randomized cooperative trial (COSS-82) with salvage chemotherapy based on histological tumor response. J Clin Oncol 6:329–337
Coldman AJ, Goldie JH (1987) Impact of dose-intense chemotherapy on the development of permanent drug resistance. Semin Oncol 14:29–33
Silber JH, Kaizer H (1988) Marginal analysis applied to the dose-response curve. Med Pediatr Oncol 16:344–348
Brown DC, Purushotham AD, Birnie GD, George WD (1995) Detection of intraoperative tumor cell dissemination in patients with breast cancer by use of reverse transcription and polymerase chain reaction. Surgery 1:96–101
Benjamin RS (1997) Freidreich’s laws in the treatment of sarcomas. Clin Cancer Res 3:2648–2654
Price LA, Hill BT (1981) Safe and effective combination chemotherapy without cis-platinum for squamous cell carcinomas of the head and neck. Cancer Treat Rep 65(Suppl 1):149S–154S
Cohen IJ (2009) High dose methotrexate is effective in osteosarcoma so what is the problem? J Pediatr Hematol Oncol 31:892–893
Cohen IJ, Wolff JE (2014) How long can folinic acid rescue be delayed after high-dose methotrexate without toxicity? Pediatr Blood Cancer 61:7–10
Bonda-Shkedi E, Arush MW, Kaplinsky C et al (2013) The correlation between dose of folinic acid and neurotoxicity in children and adolescents treated for osteosarcoma with high-dose methotrexate (HDMTX): a neuropsychological and psychosocial study. J Pediatr Hematol Oncol 35:271–275
Cohen IJ (2013) Challenging the clinical relevance of folinic acid over rescue after high dose methotrexate (HDMTX). Med Hypotheses 81:942–947
Cohen IJ (2004) Defining the appropriate dosage of folinic acid after high-dose methotrexate for childhood acute lymphatic leukemia that will prevent neurotoxicity without rescuing malignant cells in the central nervous system. J Pediatr Hematol Oncol 26:156–163
Janeway KA, Grier HE (2010) Sequelae of osteosarcoma medical therapy: a review of rare acute toxicities and late effects. Lancet Oncol 11:670–678
Ozols RF, Corden BJ, Jacob J, Wesley MN, Ostchega Y, Young RC (1984) High-dose cisplatin in hypertonic saline. Ann Intern Med 100:19–24
Ozols DF, Deisseroth AB, Javadpour N, Barlock A, Messerschmidt GL, Young RC (1983) Treatment of poor prognosis nonseminomatous testicular cancer with a “high-dose” platinum combination chemotherapy regimen. Cancer 51:1803–1807
Nagarajan R, Kamruzzaman A, Ness KK et al (2011) Twenty years of follow-up of survivors of childhood osteosarcoma: a report from the Childhood Cancer Survivor Study. Cancer 117:625–634
Gupta AA, Capra M, Papaioannou V et al (2006) Low incidence of ototoxicity with continuous infusion of cisplatin in the treatment of pediatric germ cell tumors. J Pediatr Hematol Oncol 28:91–94
Petersen PM, Hansen SW, Giwercman A, Rørth M, Skakkebaek NE (1994) Dose-dependent impairment of testicular function in patients treated with cisplatin-based chemotherapy for germ cell cancer. Ann Oncol 5:355–358
Meistrich ML, Chawla SP, Da Cunha MF et al (1989) Recovery of sperm production after chemotherapy for osteosarcoma. Cancer 63:2115–2123
Ash BS, Cohen I, Goshen Y et al (2009) Fertility in female survivors of nonmetastatic osteosarcoma. ESMO (Abstract). J Bone Joint Surg Br Proceedings 10.1302. http://www.bjj.boneandjoint.org.uk/. Accessed 1 Dec 2014
Walker DM, Fisher BT, Seif AE et al (2013) Dexrazoxane use in pediatric patients with acute lymphoblastic or myeloid leukemia from 1999 and 2009: analysis of a national cohort of patients in the Pediatric Health Information Systems database. Pediatr Blood Cancer 60:616–620
Vrooman LM, Neuberg DS, Stevenson KE et al (2011) The low incidence of secondary acute myelogenous leukaemia in children and adolescents treated with dexrazoxane for acute lymphoblastic leukaemia: a report from the Dana-Farber Cancer Institute ALL Consortium. Eur J Cancer 47:1373–1379
Tebbi CK, London WB, Friedman D et al (2007) Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin’s disease. J Clin Oncol 25:493–500
Crews KR, Liu T, Rodriguez-Galindo C et al (2004) High-dose methotrexate pharmacokinetics and outcome of children and young adults with osteosarcoma. Cancer 100:1724–1733
Meyers PA, Schwartz CL, Krailo M et al (2005) Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol 23:2004–2011
Ferrari S, Ruggieri P, Cefalo G et al (2012) Neoadjuvant chemotherapy with methotrexate, cisplatin, and doxorubicin with or without ifosfamide in nonmetastatic osteosarcoma of the extremity: an Italian sarcoma group trial ISG/OS-1. J Clin Oncol 30:2112–2118
Kudawara I, Aoki Y, Ueda T et al (2013) Neoadjuvant and adjuvant chemotherapy with high-dose ifosfamide, doxorubicin, cisplatin and high-dose methotrexate in non-metastatic osteosarcoma of the extremities: a phase II trial in Japan. J Chemother 25:41–48
Marina N, Smeland S, Bielack SS, Bernstein M, Jovic G, Hook JM, Krailo MD (2014) MAPIE vs MAP as postoperative chemotherapy in patients with a poor response to preoperative chemotherapy for newly diagnosed osteosarcoma: results from EURAMOS-1 (Paper 032). Presented at: 19th annual Connective Tissue Oncology Meeting, October 15–18, 2014, Berlin, Germany
Allison DC, Carney SC, Ahlmann ER et al (2012) A meta-analysis of osteosarcoma outcomes in modern medical era. Sarcoma 2012:10. doi:10.1155/2012/704872
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional local ethics committee and the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Shkalim-Zemer, V., Ash, S., Toledano, H. et al. Highly effective reduced toxicity dose-intensive pilot protocol for non-metastatic limb osteogenic sarcoma (SCOS 89). Cancer Chemother Pharmacol 76, 909–916 (2015). https://doi.org/10.1007/s00280-015-2865-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2865-x