Abstract
Purpose
Fluoropyrimidines and oxaliplatin have demonstrated some efficacy against pancreatic adenocarcinoma, but survival remains brief. Sorafenib is an oral multikinase inhibitor which we sought to combine with a unique capecitabine and oxaliplatin regimen for pancreatic adenocarcinoma.
Methods
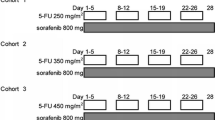
We performed a multicenter phase II study of sorafenib 200 mg orally twice daily along with oxaliplatin 85 mg/m2 IV on days 1 and 15, followed by capecitabine 2250 mg/m2 orally every 8 h for six doses starting on days 1 and 15 of a 28-day cycle in patients who had no more than one previous chemotherapy regimen for their pancreatic adenocarcinoma. The primary objective was response rate; secondary objectives were progression-free survival (PFS), overall survival (OS), and safety.
Results
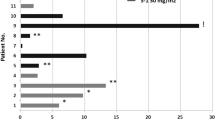
Twenty-four patients were enrolled; median age was 63 years (range 48–83). The most common related toxicities were fatigue, neuropathy, anemia, thrombocytopenia, diarrhea, nausea, leukopenia, and hand-foot syndrome. Grade 3 hand-foot syndrome was rare (4 %). Other grade 4 toxicities included abdominal pain (8 %), pulmonary embolism (4 %), and anemia (4 %). Three partial responses were seen (13 %), and 11 patients had stable disease (46 %) as their best response. Median PFS was 6.0 months (range 1.5–13 months). Median OS was 8.1 months (range 1.5–13.6 months).
Conclusions
Sorafenib, oxaliplatin, and capecitabine produced partial responses in patients with advanced pancreatic cancer including previously treated patients and demonstrated a PFS of 6 months with few grade 3/4 toxicities.



Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin. doi:10.3322/caac.21254
Von Hoff DD, Ervin T, Arena FP et al (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369:1691–1703. doi:10.1056/NEJMoa1304369
Conroy T, Desseigne F, Ychou M et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825. doi:10.1056/NEJMoa1011923
Cheeseman SL, Joel SP, Chester JD et al (2002) A “modified de Gramont” regimen of fluorouracil, alone and with oxaliplatin, for advanced colorectal cancer. Br J Cancer 87:393–399. doi:10.1038/sj.bjc.6600467
Scheithauer W, Kornek GV, Raderer M et al (2002) Intermittent weekly high-dose capecitabine in combination with oxaliplatin: a phase I/II study in first-line treatment of patients with advanced colorectal cancer. Ann Oncol 13:1583–1589
Van Cutsem E, Hoff PM, Harper P et al (2004) Oral capecitabine vs intravenous 5-fluorouracil and leucovorin: integrated efficacy data and novel analyses from two large, randomised, phase III trials. Br J Cancer 90:1190–1197. doi:10.1038/sj.bjc.6601676
Mulkerin D, LoConte NK, Holen KD et al (2009) A phase I study of an oral simulated FOLFOX with high dose capecitabine. Invest New Drugs 27:461–468. doi:10.1007/s10637-008-9210-8
Wilhelm SM, Adnane L, Newell P et al (2008) Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther 7:3129–3140. doi:10.1158/1535-7163.MCT-08-0013
Hruban RH, van Mansfeld AD, Offerhaus GJ et al (1993) K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol 143:545–554
Wilhelm SM, Carter C, Tang L et al (2004) BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64:7099–7109. doi:10.1158/0008-5472.CAN-04-1443
Ulivi P, Arienti C, Amadori D et al (2009) Role of RAF/MEK/ERK pathway, p-STAT-3 and Mcl-1 in sorafenib activity in human pancreatic cancer cell lines. J Cell Physiol 220:214–221. doi:10.1002/jcp.21753
Escudier B, Eisen T, Stadler WM et al (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356:125–134. doi:10.1056/NEJMoa060655
Lubner SJ, Loconte NK, Holen KD et al (2010) A phase II study of oxaliplatin, 5-fluorouracil, leucovorin, and high-dose capecitabine in patients with metastatic colorectal cancer. Clin Colorectal Cancer 9:157–161. doi:10.3816/CCC.2010.n.021
LoConte NK, Holen KD, Schelman WR et al (2013) A phase I study of sorafenib, oxaliplatin and 2 days of high dose capecitabine in advanced pancreatic and biliary tract cancer: a Wisconsin oncology network study. Invest New Drugs 31:943–948. doi:10.1007/s10637-012-9916-5
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. doi:10.1016/j.ejca.2008.10.026
CTEP (2006) Common Terminology Criteria for Adverse Events v3.0 (CTCAE). http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed 9 June 2015
Rahma OE, Duffy A, Liewehr DJ et al (2013) Second-line treatment in advanced pancreatic cancer: a comprehensive analysis of published clinical trials. Ann Oncol 24:1972–1979. doi:10.1093/annonc/mdt166
Berk V, Ozdemir N, Ozkan M et al (2012) XELOX vs. FOLFOX4 as second line chemotherapy in advanced pancreatic cancer. Hepatogastroenterology 59:2635–2639. doi:10.5754/hge12181
Cartwright TH (2014) Use of first-line chemotherapy for advanced pancreatic cancer: FOLFIRINOX versus gemcitabine-based therapy. J Clin Oncol 32:5s (suppl; abstr 4132)
Zaanan A, Trouilloud I, Markoutsaki T et al (2014) FOLFOX as second-line chemotherapy in patients with pretreated metastatic pancreatic cancer from the FIRGEM study. BMC Cancer 14:441. doi:10.1186/1471-2407-14-441
Yoo C, Hwang JY, Kim J-E et al (2009) A randomised phase II study of modified FOLFIRI.3 vs modified FOLFOX as second-line therapy in patients with gemcitabine-refractory advanced pancreatic cancer. Br J Cancer 101:1658–1663. doi:10.1038/sj.bjc.6605374
Baselga J, Segalla JGM, Roché H et al (2012) Sorafenib in combination with capecitabine: an oral regimen for patients with HER2-negative locally advanced or metastatic breast cancer. J Clin Oncol 30:1484–1491. doi:10.1200/JCO.2011.36.7771
Tabernero J, Garcia-Carbonero R, Cassidy J et al (2013) Sorafenib in combination with oxaliplatin, leucovorin, and fluorouracil (modified FOLFOX6) as first-line treatment of metastatic colorectal cancer: the RESPECT trial. Clin Cancer Res 19:2541–2550. doi:10.1158/1078-0432.CCR-13-0107
Lévy E, Piedbois P, Buyse M et al (1998) Toxicity of fluorouracil in patients with advanced colorectal cancer: effect of administration schedule and prognostic factors. J Clin Oncol 16:3537–3541
Gonçalves A, Gilabert M, François E et al (2012) BAYPAN study: a double-blind phase III randomized trial comparing gemcitabine plus sorafenib and gemcitabine plus placebo in patients with advanced pancreatic cancer. Ann Oncol 23:2799–2805. doi:10.1093/annonc/mds135
Cascinu S, Berardi R, Sobrero A et al (2014) Sorafenib does not improve efficacy of chemotherapy in advanced pancreatic cancer: a GISCAD randomized phase II study. Dig Liver Dis 46:182–186. doi:10.1016/j.dld.2013.09.020
Acknowledgments
This study was funded by Sanofi-aventis, Bayer/Onyx pharmaceuticals, and University of Wisconsin Carbone Cancer Center Support Grant P30 CA014520.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Makielski, R.J., Lubner, S.J., Mulkerin, D.L. et al. A phase II study of sorafenib, oxaliplatin, and 2 days of high-dose capecitabine in advanced pancreas cancer. Cancer Chemother Pharmacol 76, 317–323 (2015). https://doi.org/10.1007/s00280-015-2783-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2783-y