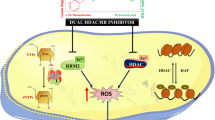
Abstract
Purpose
New (4-aryl-1-methylimidazol-5-yl)cinnamoylhydroxamic acids were prepared as potential dual mode anticancer agents combining the antivascular effect of the 4,5-diarylimidazole moiety and the histone deacetylases (HDAC) inhibition by the cinnamoyl hydroxamate.
Methods
Their antiproliferative activity against a panel of primary cells and cancer cell lines was determined by MTT assays and their apoptosis induction by caspase-3 activation. Their ability to reduce the activity of HDAC was measured by enzymatic assays and Western blot analyses of cellular HDAC substrates. Additional effects on cancer cell migration were ascertained via immunofluorescence staining of cytoskeleton components and three-dimensional migration assays. The chorioallantoic membrane assay was used as an in vivo model to assess their antiangiogenic properties.
Results
The 4-phenyl- and 4-(p-methoxyphenyl)-imidazole derivatives had a greater antiproliferative and apoptosis inducing effect in a variety of cancer cell lines when compared with the approved HDAC inhibitor SAHA, and most distinctly so in non-malignant human umbilical vein endothelial cells. Like SAHA, both compounds acted as pan-HDAC inhibitors. In 518A2 melanoma cells, they led to hyperacetylation of histones and of the cytoplasmic HDAC6 substrate alpha-tubulin. As a consequence, they inhibited the migration and invasion of these cells in transwell invasion assays. In keeping with its pronounced impact on endothelial cells, the 4-phenyl-imidazole derivative also inhibited the growth and sprouting of blood vessels in the chorioallantoic membrane of fertilized hen eggs.
Conclusions
The 4-phenyl- and 4-(p-methoxyphenyl)-imidazole compounds combine the antivascular effects of 4,5-diarylimidazoles with HDAC inhibition by cinnamoyl hydroxamates and show additional antimetastatic activity. They are promising candidates for pleiotropic HDAC inhibitors.





Similar content being viewed by others
References
Nebbioso A, Carafa V, Benedetti R, Altucci L (2012) Trials with “epigenetic” drugs: an update. Mol Oncol 6:657–682. doi:10.1016/j.molonc.2012.09.004
New M, Olzscha H, La Thangue NB (2012) HDAC inhibitor-based therapies: can we interpret the code? Mol Oncol 6:637–656. doi:10.1016/j.molonc.2012.09.003
Glozak MA, Seto E (2007) Histone deacetylases and cancer. Oncogene 26:5420–5432. doi:10.1038/sj.onc.1210610
Aldana-Masangkay GI, Sakamoto KM (2011) The role of HDAC6 in cancer. J Biomed Biotechnol 2011:1–10. doi:10.1155/2011/875824
Vidali G, Boffa LC, Bradbury EM, Allfrey VG (1978) Butyrate suppression of histone deacetylation leads to accumulation of multiacetylated forms of histones H3 and H4 and increased DNase I sensitivity of the associated DNA sequences. Proc Natl Acad Sci 75:2239–2243
Minucci S, Pelicci PG (2006) Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer 6:38–51. doi:10.1038/nrc1779
Paris M, Porcelloni M, Binaschi M, Fattori D (2008) Histone deacetylase inhibitors: from bench to clinic. J Med Chem 51:1505–1529. doi:10.1021/jm7011408
Mai A, Altucci L (2009) Epi-drugs to fight cancer: from chemistry to cancer treatment, the road ahead. Int J Biochem Cell Biol 41:199–213. doi:10.1016/j.biocel.2008.08.020
Dokmanovic M, Clarke C, Marks PA (2007) Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res 5:981–989. doi:10.1158/1541-7786.MCR-07-0324
Richon VM, Emiliani S, Verdin E et al (1998) A class of hybrid polar inducers of transformed cell differentiation inhibits histone deacetylases. Proc Natl Acad Sci 95:3003–3007
Marks PA (2007) Discovery and development of SAHA as an anticancer agent. Oncogene 26:1351–1356. doi:10.1038/sj.onc.1210204
Maiso P, Carvajal-Vergara X, Ocio EM et al (2006) The histone deacetylase inhibitor LBH589 is a potent antimyeloma agent that overcomes drug resistance. Cancer Res 66:5781–5789
Plumb JA, Finn PW, Williams RJ et al (2003) Pharmacodynamic response and inhibition of growth of human tumor xenografts by the novel histone deacetylase inhibitor PXD101. Mol Cancer Ther 2:721–728
Ellis L, Hammers H, Pili R (2009) Targeting tumor angiogenesis with histone deacetylase inhibitors. Cancer Lett 280:145–153. doi:10.1016/j.canlet.2008.11.012
Kim H-J, Bae S-C (2011) Histone deacetylase inhibitors: molecular mechanisms of action and clinical trials as anti-cancer drugs. Am J Transl Res 3:166
Qian DZ (2006) Targeting tumor angiogenesis with histone deacetylase inhibitors: the hydroxamic acid derivative LBH589. Clin Cancer Res 12:634–642. doi:10.1158/1078-0432.CCR-05-1132
Fantin VR, Richon VM (2007) Mechanisms of resistance to histone deacetylase inhibitors and their therapeutic implications. Clin Cancer Res 13:7237–7242. doi:10.1158/1078-0432.CCR-07-2114
Wang J, Pursell NW, Samson MES et al (2013) Potential advantages of CUDC-101, a multitargeted HDAC, EGFR, and HER2 inhibitor, in treating drug resistance and preventing cancer cell migration and invasion. Mol Cancer Ther 12:925–936. doi:10.1158/1535-7163.MCT-12-1045
Mahboobi S, Dove S, Sellmer A et al (2009) Design of chimeric histone deacetylase- and tyrosine kinase-inhibitors: a series of imatinib hybrides as potent inhibitors of wild-type and mutant BCR-ABL, PDGF-Rβ, and histone deacetylases. J Med Chem 52:2265–2279. doi:10.1021/jm800988r
Gryder BE, Rood MK, Johnson KA et al (2013) Histone deacetylase inhibitors equipped with estrogen receptor modulation activity. J Med Chem 56:5782–5796. doi:10.1021/jm400467w
Guerrant W, Patil V, Canzoneri JC et al (2013) Dual-acting histone deacetylase-topoisomerase I inhibitors. Bioorg Med Chem Lett 23:3283–3287. doi:10.1016/j.bmcl.2013.03.108
Biersack B, Effenberger K, Schobert R, Ocker M (2010) Oxazole-bridged combretastatin a analogues with improved anticancer properties. ChemMedChem 5:420–427. doi:10.1002/cmdc.200900477
Schobert R, Biersack B, Dietrich A et al (2010) 4-(3-Halo/amino-4,5-dimethoxyphenyl)-5-aryloxazoles and -N-methylimidazoles that are cytotoxic against combretastatin a resistant tumor cells and vascular disrupting in a cisplatin resistant germ cell tumor model. J Med Chem 53:6595–6602. doi:10.1021/jm100345r
Di Fazio P, Lingelbach S, Schobert R, Biersack B (2014) 4,5-Diaryl imidazoles with hydroxamic acid appendages as anti-hepatoma agents. Invest New Drugs. doi:10.1007/s10637-014-0188-0
Shimada Y, Imamura M, Wagata T et al (1992) Characterization of 21 newly established esophageal cancer cell lines. Cancer 69:277–284
Sutter AP, Höpfner M, Huether A et al (2006) Targeting the epidermal growth factor receptor by erlotinib (Tarceva™) for the treatment of esophageal cancer. Int J Cancer 118:1814–1822. doi:10.1002/ijc.21512
Evers BM, Ishizuka J, Townsend CM, Thompson JC (1994) The human carcinoid cell line, BON: a model system for the study of carcinoid tumors. Ann N Y Acad Sci 733:393–406. doi:10.1111/j.1749-6632.1994.tb17289.x
Gloesenkamp CR, Nitzsche B, Ocker M et al (2011) AKT inhibition by triciribine alone or as combination therapy for growth control of gastroenteropancreatic neuroendocrine tumors. Int J Oncol 40:876–888. doi:10.3892/ijo.2011.1256
Gloesenkamp C, Nitzsche B, Lim AR et al (2012) Heat shock protein 90 is a promising target for effective growth inhibition of gastrointestinal neuroendocrine tumors. Int J Oncol 40:1659–1667. doi:10.3892/ijo.2012.1328
Hofmann UB, Houben R, Bröcker E-B, Becker JC (2005) Role of matrix metalloproteinases in melanoma cell invasion. Biochimie 87:307–314. doi:10.1016/j.biochi.2005.01.013
Boyden S (1962) The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med 115:453–466
Entschladen F, Drell TL, Lang K et al (2005) Analysis methods of human cell migration. Exp Cell Res 307:418–426. doi:10.1016/j.yexcr.2005.03.029
Albini A, Iwamoto Y, Kleinman HK et al (1987) A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res 47:3239–3245
Nitzsche B, Gloesenkamp C, Schrader M et al (2010) Novel compounds with antiangiogenic and antiproliferative potency for growth control of testicular germ cell tumours. Br J Cancer 103:18–28. doi:10.1038/sj.bjc.6605725
Baradari V, Huether A, Hopfner M et al (2006) Antiproliferative and proapoptotic effects of histone deacetylase inhibitors on gastrointestinal neuroendocrine tumor cells. Endocr Relat Cancer 13:1237–1250. doi:10.1677/erc.1.01249
Kim MS, Yamashita K, Baek JH et al (2006) N-methyl-d-aspartate receptor type 2B is epigenetically inactivated and exhibits tumor-suppressive activity in human esophageal cancer. Cancer Res 66:3409–3418
Zhang Y, Li N, Caron C et al (2003) HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. EMBO J 22:1168–1179
Hubbert C, Guardiola A, Shao R et al (2002) HDAC6 is a microtubule-associated deacetylase. Nature 417:455–458. doi:10.1038/417455a
Matsuyama A, Shimazu T, Sumida Y et al (2002) In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J 21:6820–6831
Sadoul K, Wang J, Diagouraga B, Khochbin S (2011) The tale of protein lysine acetylation in the cytoplasm. J Biomed Biotechnol 2011:1–15. doi:10.1155/2011/970382
Kim SC, Sprung R, Chen Y et al (2006) Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell 23:607–618. doi:10.1016/j.molcel.2006.06.026
Zhang X, Yuan Z, Zhang Y et al (2007) HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol Cell 27:197–213. doi:10.1016/j.molcel.2007.05.033
Friedl P, Wolf K (2003) Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 3:362–374. doi:10.1038/nrc1075
Deroanne CF, Bonjean C, Servotte S et al (2002) Histone deacetylases inhibitors as anti-angiogenic agents altering vascular endothelial growth factor signaling. Oncogene 21:427–436
Jeong J-W, Bae M-K, Ahn M-Y et al (2002) Regulation and destabilization of HIF-1α by ARD1-mediated acetylation. Cell 111:709–720
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
280_2015_2685_MOESM1_ESM.doc
The online version of this article contains supplementary material (synthesis and compound characterization; additional method descriptions and data; original Western blot images), which is available to authorized users 1 (DOC 3705 kb)
Rights and permissions
About this article
Cite this article
Mahal, K., Schruefer, S., Steinemann, G. et al. Biological evaluation of 4,5-diarylimidazoles with hydroxamic acid appendages as novel dual mode anticancer agents. Cancer Chemother Pharmacol 75, 691–700 (2015). https://doi.org/10.1007/s00280-015-2685-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2685-z