Abstract
Purpose
Through enhancement of 6-mercaptopurine (6MP) bioavailability and inhibition of purine de novo synthesis, high-dose methotrexate (HD-MTX) may increase incorporation into DNA of 6-thioguanine nucleotides, the cytotoxic metabolites of 6MP. Patients with intermediate activity of thiopurine methyltransferase (TPMTIA) have higher cytosol 6-thioguanine nucleotide levels. We investigated toxicity following HD-MTX during MTX/6MP maintenance therapy in relation to 6MP and TPMT.
Methods
Using linear mixed models, we explored myelo- and hepatotoxicity in relation to 6MP dosage and TPMT phenotype following 1,749 HD-MTX courses to 411 children with acute lymphoblastic leukemia on maintenance therapy.
Results
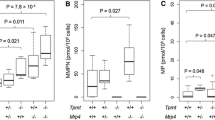
The degree of myelosuppression following HD-MTX was similar for patients with TPMTIA and patients with high TPMT activity (TPMTHA), when HD-MTX started with same blood counts and 6MP doses. However, since TPMTIA had lower blood counts at initiation of HD-MTX compared with TPMTHA patients (median WBC 2.8 vs. 3.3 × 109/L, P = 0.01; median ANC 1.4 vs. 1.7 × 109/L, P = 0.02), TPMTIA continued to have lower WBC and ANC levels compared with TPMTHA during all 28 days after HD-MTX [relative difference 9 % (95 % CI 2–17), P = 0.02 and 21 % (95 % CI 6–39), P = 0.005]. Still, the fractional decrease in WBC and ANC levels after HD-MTX did not differ between TPMTIA and TPMTHA patients (P = 0.47; P = 0.38). The degree of leukopenia, neutropenia, thrombocytopenia and rise in aminotransferases were all significantly related to 6MP dose (P < 0.001 for all analyses).
Conclusion
For both TPMTIA and TPMTHA patients, dose of 6MP prior to HD-MTX should be guided by pre-HD-MTX blood counts, but not by TPMT activity.




Similar content being viewed by others
References
Schrappe M, Nachman J, Hunger S, Schmiegelow K, Conter V, Masera G, Pieters R, Pui CH (2010) Educational symposium on long-term results of large prospective clinical trials for childhood acute lymphoblastic leukemia (1985–2000). Leukemia 24:253–254
Clarke M, Gaynon P, Hann I, Harrison G, Masera G, Peto R, Richards S (2003) CNS-directed therapy for childhood acute lymphoblastic leukemia: Childhood ALL Collaborative Group overview of 43 randomized trials. J Clin Oncol 21:1798–1809
Pui CH, Thiel E (2009) Central nervous system disease in hematologic malignancies: historical perspective and practical applications. Semin Oncol 36:S2–S16
Nathan PC, Whitcomb T, Wolters PL, Steinberg SM, Balis FM, Brouwers P, Hunsberger S, Feusner J, Sather H, Miser J, Odom LF, Poplack D, Reaman G, Bleyer WA (2006) Very high-dose methotrexate (33.6 g/m2) as central nervous system preventive therapy for childhood acute lymphoblastic leukemia: results of National Cancer Institute/Children’s Cancer Group trials CCG-191P, CCG-134P and CCG-144P. Leuk Lymphoma 47:2488–2504
Pui CH, Evans WE (1998) Acute lymphoblastic leukemia. N Engl J Med 339:605–615
Skarby TV, Anderson H, Heldrup J, Kanerva JA, Seidel H, Schmiegelow K (2006) High leucovorin doses during high-dose methotrexate treatment may reduce the cure rate in childhood acute lymphoblastic leukemia. Leukemia 20:1955–1962
Rask C, Albertioni F, Bentzen SM, Schroeder H, Peterson C (1998) Clinical and pharmacokinetic risk factors for high-dose methotrexate-induced toxicity in children with acute lymphoblastic leukemia—a logistic regression analysis. Acta Oncol 37:277–284
Peeters M, Koren G, Jakubovicz D, Zipursky A (1988) Physician compliance and relapse rates of acute lymphoblastic leukemia in children. Clin Pharmacol Ther 43:228–232
Relling MV, Hancock ML, Boyett JM, Pui CH, Evans WE (1999) Prognostic importance of 6-mercaptopurine dose intensity in acute lymphoblastic leukemia. Blood 93:2817–2823
Schmiegelow K (1991) Prognostic significance of methotrexate and 6-mercaptopurine dosage during maintenance chemotherapy for childhood acute lymphoblastic leukemia. Pediatr Hematol Oncol 8:301–312
Balis FM, Holcenberg JS, Zimm S, Tubergen D, Collins JM, Murphy RF, Gilchrist GS, Hammond D, Poplack DG (1987) The effect of methotrexate on the bioavailability of oral 6-mercaptopurine. Clin Pharmacol Ther 41:384–387
Bokkerink JP, Bakker MA, Hulscher TW, De Abreu RA, Schretlen ED (1988) Purine de novo synthesis as the basis of synergism of methotrexate and 6-mercaptopurine in human malignant lymphoblasts of different lineages. Biochem Pharmacol 37:2321–2327
Giverhaug T, Loennechen T, Aarbakke J (1999) The interaction of 6-mercaptopurine (6-MP) and methotrexate (MTX). Gen Pharmacol 33:341–346
Innocenti F, Danesi R, Di PA, Loru B, Favre C, Nardi M, Bocci G, Nardini D, Macchia P, Del TM (1996) Clinical and experimental pharmacokinetic interaction between 6-mercaptopurine and methotrexate. Cancer Chemother Pharmacol 37:409–414
Schmiegelow K, Bretton-Meyer U (2001) 6-mercaptopurine dosage and pharmacokinetics influence the degree of bone marrow toxicity following high-dose methotrexate in children with acute lymphoblastic leukemia. Leukemia 15:74–79
van Kooten Niekerk PB, Schmiegelow K, Schroeder H (2008) Influence of methylene tetrahydrofolate reductase polymorphisms and coadministration of antimetabolites on toxicity after high dose methotrexate. Eur J Haematol 81:391–398
Nygaard U, Schmiegelow K (2003) Dose reduction of coadministered 6-mercaptopurine decreases myelotoxicity following high-dose methotrexate in childhood leukemia. Leukemia 17:1344–1348
Karran P, Attard N (2008) Thiopurines in current medical practice: molecular mechanisms and contributions to therapy-related cancer. Nat Rev Cancer 8:24–36
Duley JA, Florin TH (2005) Thiopurine therapies: problems, complexities, and progress with monitoring thioguanine nucleotides. Ther Drug Monit 27:647–654
Weinshilboum RM, Otterness DM, Szumlanski CL (1999) Methylation pharmacogenetics: catechol O-methyltransferase, thiopurine methyltransferase, and histamine N-methyltransferase. Annu Rev Pharmacol Toxicol 39:19–52
Wang L, Weinshilboum R (2006) Thiopurine S-methyltransferase pharmacogenetics: insights, challenges and future directions. Oncogene 25:1629–1638
Ebbesen MS, Nersting J, Jacobsen JH, Frandsen TL, Vettenranta K, Abramsson J, Wesenberg F, Schmiegelow K (2013) Incorporation of 6-thioguanine nucleotides into DNA during maintenance therapy of childhood acute lymphoblastic leukemia—the influence of thiopurine methyltransferase genotypes. J Clin Pharmacol 53:670–674
Schmiegelow K, Bjork O, Glomstein A, Gustafsson G, Keiding N, Kristinsson J, Makipernaa A, Rosthoj S, Szumlanski C, Sorensen TM, Weinshilboum R (2003) Intensification of mercaptopurine/methotrexate maintenance chemotherapy may increase the risk of relapse for some children with acute lymphoblastic leukemia. J Clin Oncol 21:1332–1339
Schmiegelow K, Forestier E, Kristinsson J, Soderhall S, Vettenranta K, Weinshilboum R, Wesenberg F (2009) Thiopurine methyltransferase activity is related to the risk of relapse of childhood acute lymphoblastic leukemia: results from the NOPHO ALL-92 study. Leukemia 23:557–564
Andersen JB, Szumlanski C, Weinshilboum RM, Schmiegelow K (1998) Pharmacokinetics, dose adjustments, and 6-mercaptopurine/methotrexate drug interactions in two patients with thiopurine methyltransferase deficiency. Acta Paediatr 87:108–111
Gustafsson G, Schmiegelow K, Forestier E, Clausen N, Glomstein A, Jonmundsson G, Mellander L, Makipernaa A, Nygaard R, Saarinen-Pihkala UM (2000) Improving outcome through two decades in childhood ALL in the Nordic countries: the impact of high-dose methotrexate in the reduction of CNS irradiation. Nordic Society of Pediatric Haematology and Oncology (NOPHO). Leukemia 14:2267–2275
Kaplan EJ, Meier P (1958) Non-parametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Mantel N (1966) Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother 50:163–170
Liang KY, Zeger SL (1986) Longitudinal data analysis using generalized linear models. Biometrika 73:13–22
Pesarin F, Salmaso L (2010) Permutation tests for complex data: theory, applications and software. Wiley, Chicester
Pinheiro JC, Bates DM (2000) Mixed-effect models in S and S-Plus. Springer, New York
Harrell FE (2001) Regression modeling strategies: with applications to linear models, logistic regression and survival analysis. Springer, New York
Efron B (1981) Nonparametric estimates of standard error: the jackknife, the bootstrap and other methods. Biometrika 68:589–599
Schmiegelow K (2009) Advances in individual prediction of methotrexate toxicity: a review. Br J Haematol 146:489–503
Evans WE, Horner M, Chu YQ, Kalwinsky D, Roberts WM (1991) Altered mercaptopurine metabolism, toxic effects, and dosage requirement in a thiopurine methyltransferase-deficient child with acute lymphocytic leukemia. J Pediatr 119:985–989
Lennard L, Gibson BE, Nicole T, Lilleyman JS (1993) Congenital thiopurine methyltransferase deficiency and 6-mercaptopurine toxicity during treatment for acute lymphoblastic leukaemia. Arch Dis Child 69:577–579
McLeod HL, Coulthard S, Thomas AE, Pritchard SC, King DJ, Richards SM, Eden OB, Hall AG, Gibson BE (1999) Analysis of thiopurine methyltransferase variant alleles in childhood acute lymphoblastic leukaemia. Br J Haematol 105:696–700
McLeod HL, Krynetski EY, Relling MV, Evans WE (2000) Genetic polymorphism of thiopurine methyltransferase and its clinical relevance for childhood acute lymphoblastic leukemia. Leukemia 14:567–572
Relling MV, Hancock ML, Rivera GK, Sandlund JT, Ribeiro RC, Krynetski EY, Pui CH, Evans WE (1999) Mercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus. J Natl Cancer Inst 91:2001–2008
Schmiegelow K, Al-Modhwahi I, Andersen MK, Behrendtz M, Forestier E, Hasle H, Heyman M, Kristinsson J, Nersting J, Nygaard R, Svendsen AL, Vettenranta K, Weinshilboum R (2009) Methotrexate/6-mercaptopurine maintenance therapy influences the risk of a second malignant neoplasm after childhood acute lymphoblastic leukemia: results from the NOPHO ALL-92 study. Blood 113:6077–6084
Levinsen M, Rotevatn EO, Rosthoj S, Nersting J, Abrahamsson J, Appell ML, Bergan S, Bechensteen AG, Harila-Saari A, Heyman M, Jonsson OG, Maxild JB, Niemi M, Soderhall S, Schmiegelow K (2014) Pharmacogenetically based dosing of thiopurines in childhood acute lymphoblastic leukemia: influence on cure rates and risk of second cancer. Pediatr Blood Cancer 61:797–802
Stanulla M, Schaeffeler E, Moricke A, Coulthard SA, Cario G, Schrauder A, Kaatsch P, Dordelmann M, Welte K, Zimmermann M, Reiter A, Eichelbaum M, Riehm H, Schrappe M, Schwab M (2009) Thiopurine methyltransferase genetics is not a major risk factor for secondary malignant neoplasms after treatment of childhood acute lymphoblastic leukemia on Berlin–Frankfurt–Munster protocols. Blood 114:1314–1318
Coulthard SA, Howell C, Robson J, Hall AG (1998) The relationship between thiopurine methyltransferase activity and genotype in blasts from patients with acute leukemia. Blood 92:2856–2862
Lennard L (2014) Implementation of TPMT testing. Br J Clin Pharmacol 77:704–714
Schaeffeler E, Fischer C, Brockmeier D, Wernet D, Moerike K, Eichelbaum M, Zanger UM, Schwab M (2004) Comprehensive analysis of thiopurine S-methyltransferase phenotype–genotype correlation in a large population of German-Caucasians and identification of novel TPMT variants. Pharmacogenetics 14:407–417
Acknowledgments
We acknowledge the critical input of Dr. P. D. Cole. The commitment and skillful technical assistance of Michael Timm are greatly appreciated. The study was funded by The Otto Christensens Fund, The Danish Childhood Cancer foundation, The Carl and Ellen Hertz Foundation, The Children’s Cancer Foundation of Sweden, The Danish Cancer Society, The JPC Foundation, The Lundbeck Foundation, The Minister Erna Hamilton Foundation, The Nordic Cancer Union and the US National Institutes of Health Grants R01 GM28157 and U19 GM61388.
Conflict of interest
None.
Ethical standard
The ALL92 protocol was approved by the Ethical Committee of Copenhagen as well as by the local ethical committees, and participants gave informed consent according to the Helsinki Declaration.
Author information
Authors and Affiliations
Corresponding author
Additional information
For the Nordic Society of Paediatric Haematology and Oncology (NOPHO).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Levinsen, M., Rosthøj, S., Nygaard, U. et al. Myelotoxicity after high-dose methotrexate in childhood acute leukemia is influenced by 6-mercaptopurine dosing but not by intermediate thiopurine methyltransferase activity. Cancer Chemother Pharmacol 75, 59–66 (2015). https://doi.org/10.1007/s00280-014-2613-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2613-7