Abstract
Purpose
This study assessed the pharmacokinetics and safety of oral panobinostat and its metabolite BJB432 in patients with advanced solid tumors and normal to severely impaired renal function.
Methods
Patients with varying degrees of renal impairment, defined by their 24-h baseline urine creatinine clearance (as normal, mild, moderate or severe), received a single oral dose of 30 mg panobinostat. Serial plasma samples were collected pre-dose and up to 96-h post-dose. Serial urine samples were collected for 24-h post-dose. Following the serial PK sampling, patients received 30 mg oral panobinostat thrice weekly for as long as the patient had benefit. Pharmacokinetic parameters were derived using non-compartmental analysis.
Results
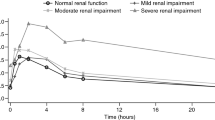
Thirty-seven patients were enrolled, and median age was 64 (range 40–81) years. Eleven patients had normal renal function; 10, 10, and 6 patients had mild, moderate, and severe renal impairment, respectively. Geometric means of AUC0–∞ in the normal, mild, moderate, and severe groups were 224.5, 144.3, 223.1, and 131.7 ng h/mL, respectively. Geometric mean ratio of BJB432 to parent drug plasma AUC0–∞ was 0.64 in the normal group and increased to 0.81, 1.13, and 1.20 in the mild, moderate, and severe groups, respectively. The fraction excreted as unchanged panobinostat was small (<2 %), with a large variability. The renal clearance of panobinostat and tolerability was similar across all four groups.
Conclusion
Systemic exposure to panobinostat did not increase with severity of renal impairment, and the drug was tolerated equally; thus, patients with renal impairment do not require starting dose adjustments.



Similar content being viewed by others

References
Atadja P (2009) Development of the pan-DAC inhibitor panobinostat (LBH589): successes and challenges. Cancer Lett 280:233–241
Lane AA, Chabner BA (2009) Histone deacetylase inhibitors in cancer therapy. J Clin Oncol 27:5459–5468
Atadja PW (2011) HDAC inhibitors and cancer therapy. Prog Drug Res 67:175–195
DeAngelo DJ, Spencer A, Bhalla KN, Prince HM, Fischer T, Kindler T et al (2013) Phase Ia/II, two-arm, open-label, dose-escalation study of oral panobinostat administered via two dosing schedules in patients with advanced hematologic malignancies. Leukemia 27:1628–1636
Ellis L, Pan Y, Smyth GK, George DJ, McCormack C, Williams-Truax R et al (2008) Histone deacetylase inhibitor panobinostat induces clinical responses with associated alterations in gene expression profiles in cutaneous T-cell lymphoma. Clin Cancer Res 14:4500–4510
Younes A, Sureda A, Ben-Yehuda D, Zinzani PL, Ong TC, Prince HM et al (2012) Panobinostat in patients with relapsed/refractory Hodgkin’s lymphoma after autologous stem-cell transplantation: results of a phase II study. J Clin Oncol 30:2197–2203
San-Miguel JF, Richardson PG, Gunther A, Sezer O, Siegel D, Blade J et al (2013) Phase ib study of panobinostat and bortezomib in relapsed or relapsed and refractory multiple myeloma. J Clin Oncol 31:3696–3703
Clive S, Woo MM, Nydam T, Kelly L, Squier M, Kagan M (2012) Characterizing the disposition, metabolism, and excretion of an orally active pan-deacetylase inhibitor, panobinostat, via trace radiolabeled 14C material in advanced cancer patients. Cancer Chemother Pharmacol 70:513–522
Wolf JL, Siegel D, Goldschmidt H, Hazell K, Bourquelot PM, Bengoudifa BR et al (2012) Phase II trial of the pan-deacetylase inhibitor panobinostat as a single agent in advanced relapsed/refractory multiple myeloma. Leuk Lymphoma 53:1820–1823
Fredenhagen A, Kittelmann M, Oberer L, Kuhn A, Kuhnol J, Delemonte T et al (2012) Biocatalytic synthesis and structure elucidation of cyclized metabolites of the deacetylase inhibitor panobinostat (LBH589). Drug Metab Dispos 40:1041–1050
Slingerland M, Guchelaar HJ, Gelderblom H (2014) Histone deacetylase inhibitors: an overview of the clinical studies in solid tumors. Anticancer Drugs 25:140–149
Anne M, Sammartino D, Barginear MF, Budman D (2013) Profile of panobinostat and its potential for treatment in solid tumors: an update. Onco Targets Ther 6:1613–1624
Khot A, Dickinson M, Prince HM (2013) Panobinostat in lymphoid and myeloid malignancies. Exp Opin Investig Drugs 22:1211–1223
Passamonti F, Maffioli M, Caramazza D (2012) New generation small-molecule inhibitors in myeloproliferative neoplasms. Curr Opin Hematol 19:117–123
Prince HM, Bishton MJ, Johnstone RW (2009) Panobinostat (LBH589): a potent pan-deacetylase inhibitor with promising activity against hematologic and solid tumors. Future Oncol 5:601–612
Slingerland M, Hess D, Clive S, Sharma S, Sandstrom P, Loman N et al (2014) A phase I, open-label, multicenter study to evaluate the pharmacokinetics and safety of oral panobinostat in patients with advanced solid tumors and various degrees of hepatic function. Cancer Chemother Pharmacol 74(5):1089–1098
de Estella-Hermoso MA, Imbuluzqueta I, Campanero MA, Gonzalez D, Vilas-Zornoza A, Agirre X et al (2011) Development and validation of ultra high performance liquid chromatography-mass spectrometry method for LBH589 in mouse plasma and tissues. J Chromatogr B Anal Technol Biomed Life Sci 879:3490–3496
Bauer S, Hilger RA, Muhlenberg T, Grabellus F, Nagarajah J, Hoiczyk M et al (2014) Phase I study of panobinostat and imatinib in patients with treatment-refractory metastatic gastrointestinal stromal tumors. Br J Cancer 110:1155–1162
Shapiro GI, Frank R, Dandamudi UB, Hengelage T, Zhao L, Gazi L et al (2012) The effect of food on the bioavailability of panobinostat, an orally active pan-histone deacetylase inhibitor, in patients with advanced cancer. Cancer Chemother Pharmacol 69:555–562
Weber HA, Tai F, Paul S, Schindler J, Woo MM, Spence S et al (2009) QT interval measurements in patients with hematologic malignancies and solid tumors treated with oral panobinostat (LBH589). ASH Annu Meet Abstr 114:3781
Ethical standard
The study was performed in accordance with ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The protocol was approved by the institutional review board at each participating institution. All patients provided written informed consent. The study is registered at www.clinicaltrials.gov with the identifier NCT00997399.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, S., Witteveen, P.O., Lolkema, M.P. et al. A phase I, open-label, multicenter study to evaluate the pharmacokinetics and safety of oral panobinostat in patients with advanced solid tumors and varying degrees of renal function. Cancer Chemother Pharmacol 75, 87–95 (2015). https://doi.org/10.1007/s00280-014-2612-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2612-8