Abstract
Purpose
Pemetrexed has shown substantial activity in non-squamous non-small cell lung cancer (NSCLC) and is one of the current standard agents in second-line settings due to its efficacy and favorable tolerability profile. We conducted phase II study to evaluate the safety and efficacy of pemetrexed in Japanese patients with previously heavily treated, advanced non-squamous NSCLC.
Methods
Patients with stage IIIB or IV non-squamous NSCLC, performance status (PS) 0–2, previous two to five regimens of chemotherapy were enrolled and received pemetrexed (500 mg/m2) on day 1 every 21 days until disease progression. The primary endpoint was progression-free survival (PFS). The secondary endpoints included overall survival (OS), objective response rate (ORR), disease control rate (DCR), and safety.
Results
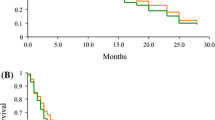
From August 2009 to May 2010, 46 patients were enrolled: median age 65 years; 52 % women; PS 0/1/2 26/67/7 %; previous treatment regimen 2/3/4/5 48/28/20/4 %; epidermal growth factor receptor activating mutation positive/wild/unknown 30/48/22 %. The median follow-up period was 13.5 months. The median number of treatment cycles was 4 (range 1–18 cycles). The median PFS was 5.2 months (95 % CI 3.0–5.8 months). The median OS was 14.4 months (95 % CI 9.4–21.3 months). The ORR was 8.7 % and DCR was 63.0 %. The grade 3/4 hematological adverse events include 8 patients with leukopenia, 11 with neutropenia, 5 with anemia, and 2 with thrombocytopenia. There were no reports of febrile neutropenia and no treatment-related death was observed.
Conclusion
Treatment with pemetrexed in previously heavily treated Japanese non-squamous NSCLC patients is feasible and shows encouraging activity.


Similar content being viewed by others
References
Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, Gatzemeier U, Tsao TC, Pless M, Muller T, Lim HL, Desch C, Szondy K, Gervais R, Shaharyar R, Manegold C, Paul S, Paoletti P, Einhorn L, Bunn PA Jr (2004) Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 22(9):1589–1597. doi:10.1200/JCO.2004.08.163
Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O’Rourke M, Levitan N, Gressot L, Vincent M, Burkes R, Coughlin S, Kim Y, Berille J (2000) Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 18(10):2095–2103
Fossella FV, DeVore R, Kerr RN, Crawford J, Natale RR, Dunphy F, Kalman L, Miller V, Lee JS, Moore M, Gandara D, Karp D, Vokes E, Kris M, Kim Y, Gamza F, Hammershaimb L (2000) Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 non-small cell lung cancer study group. J Clin Oncol 18(12):2354–2362
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabarbara P, Seymour L (2005) Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353(2):123–132. doi:10.1056/NEJMoa050753
Maruyama R, Nishiwaki Y, Tamura T, Yamamoto N, Tsuboi M, Nakagawa K, Shinkai T, Negoro S, Imamura F, Eguchi K, Takeda K, Inoue A, Tomii K, Harada M, Masuda N, Jiang H, Itoh Y, Ichinose Y, Saijo N, Fukuoka M (2008) Phase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol 26(26):4244–4252. doi:10.1200/JCO.2007.15.0185
Adjei AA (2004) Pemetrexed (ALIMTA), a novel multitargeted antineoplastic agent. Clin Cancer Res 10(12 Pt 2):4276s–4280s. doi:10.1158/1078-0432.CCR-040010
Scagliotti G, Hanna N, Fossella F, Sugarman K, Blatter J, Peterson P, Simms L, Shepherd FA (2009) The differential efficacy of pemetrexed according to NSCLC histology: a review of two Phase III studies. Oncologist 14(3):253–263. doi:10.1634/theoncologist.2008-0232
Sun JM, Lee KW, Kim JH, Kim YJ, Yoon HI, Lee JH, Lee CT, Lee JS (2009) Efficacy and toxicity of pemetrexed as a third-line treatment for non-small cell lung cancer. Jpn J Clin Oncol 39(1):27–32. doi:10.1093/jjco/hyn118
Wakelee HA, Wang W, Schiller JH, Langer CJ, Sandler AB, Belani CP, Johnson DH (2006) Survival differences by sex for patients with advanced non-small cell lung cancer on Eastern Cooperative Oncology Group trial 1594. J Thorac Oncol 1(5):441–446
Chang MH, Ahn JS, Lee J, Kim KH, Park YH, Han J, Ahn MJ, Park K (2010) The efficacy of pemetrexed as a third- or fourth-line therapy and the significance of thymidylate synthase expression in patients with advanced non-small cell lung cancer. Lung Cancer 69(3):323–329. doi:10.1016/j.lungcan.2009.12.002
Asahina H, Sekine I, Horinouchi H, Nokihara H, Yamamoto N, Kubota K, Tamura T (2012) Retrospective analysis of third-line and fourth-line chemotherapy for advanced non-small-cell lung cancer. Clin Lung Cancer 13(1):39–43. doi:10.1016/j.cllc.2011.06.010
Acknowledgments
The authors are grateful to the members of HANSHIN Oncology Group.
Conflict of interest
The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Clinical trials registration number UMIN ID: UMIN000002467.
Rights and permissions
About this article
Cite this article
Hattori, Y., Satouchi, M., Katakami, N. et al. A phase II study of pemetrexed in patients with previously heavily treated non-squamous non-small cell lung cancer (HANSHIN Oncology Group 001). Cancer Chemother Pharmacol 73, 17–23 (2014). https://doi.org/10.1007/s00280-013-2290-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2290-y