Abstract
Purpose
The aim of this study was to compare CKD-810 (test docetaxel) with Taxotere® (reference docetaxel) in terms of pharmacokinetics and safety for patients with advanced or metastatic carcinoma.
Methods
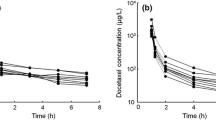
A randomized, open-label, two-way crossover study was conducted in eligible patients. Patients received with reference or test drugs of 75 mg/m2 docetaxel by intravenous infusion for 60 min in the first period and the alternative drug in the second period with a washout of 3 weeks. Plasma concentrations of docetaxel were determined by validated high-performance liquid chromatography coupled to tandem mass spectrometry detection. Pharmacokinetic parameters, including the maximum plasma concentration (C max) and the area under the concentration–time curve (AUC), were determined by non-compartmental analysis.
Results
A total of 44 patients were included in the study, 21 patients received test drug and 23 received reference drug for the first cycle. The C max of docetaxel was 2,658.77 ng/mL for test drug and 2,827.60 ng/mL for reference drug, and two drugs showed no difference with a statistical significance. Time to reach C max (T max) of CKD-810 (0.94 h) versus reference docetaxel (0.97 h) was also not significantly different. Other pharmacokinetic parameters including the plasma AUC, elimination half-life, and total body clearance exhibited similar values without a significant difference. The most common grade 3 or 4 toxicity was neutropenia (CKD-810 19.5 or 29.3 %; reference docetaxel 14.6 or 41.5 %). Febrile neutropenia was experienced by only one patient in each group. Two patients died of progression of disease during the study.
Conclusion
Docetaxel anhydrous CKD-810 use with patients suffering advanced or metastatic solid malignancies was equivalent to reference docetaxel in terms of pharmacokinetic parameters and safety profile. Additionally, the test and reference drug met the regulatory criteria for pharmacokinetic equivalence.

Similar content being viewed by others
References
Ajani JA (2006) Chemotherapy for advanced gastric or gastroesophageal cancer: defining the contributions of docetaxel. Expert Opin Pharmacother 7:1627–1631
Bissery MC, Nohynek G, Sanderink GJ, Lavelle F (1995) Docetaxel (Taxotere): a review of preclinical and clinical experience. Part I: Preclinical experience. Anticancer Drugs 6:339–355–363–338
Bouvier E, Thirot S, Schmidt F, Monneret C (2004) First enzymatically activated Taxotere prodrugs designed for ADEPT and PMT. Bioorg Med Chem 12:969–977
Diaz JF, Andreu JM (1993) Assembly of purified GDP-tubulin into microtubules induced by taxol and Taxotere: reversibility, ligand stoichiometry, and competition. Biochemistry 32:2747–2755
Gueritte-Voegelein F, Guenard D, Lavelle F, Le Goff MT, Mangatal L, Potier P (1991) Relationships between the structure of taxol analogues and their antimitotic activity. J Med Chem 34:992–998
Ringel I, Horwitz SB (1991) Studies with RP 56976 (Taxotere): a semisynthetic analogue of taxol. J Natl Cancer Inst 83:288–291
Capri G, Tarenzi E, Fulfaro F, Gianni L (1996) The role of taxanes in the treatment of breast cancer. Semin Oncol 23:68–75
Kaye SB (1995) Taxoids. Eur J Cancer 31A:824–826
Rowinsky EK (1997) The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annu Rev Med 48:353–374
Bergh M, Magnusson K, Nilsson JL, Karlberg AT (1997) Contact allergenic activity of Tween 80 before and after air exposure. Contact Dermatitis 37:9–18
Lorenz W, Schmal A, Schult H, Lang S, Ohmann C, Weber D, Kapp B, Luben L, Doenicke A (1982) Histamine release and hypotensive reactions in dogs by solubilizing agents and fatty acids: analysis of various components in cremophor El and development of a compound with reduced toxicity. Agents Actions 12:64–80
Drori S, Eytan GD, Assaraf YG (1995) Potentiation of anticancer-drug cytotoxicity by multidrug-resistance chemosensitizers involves alterations in membrane fluidity leading to increased membrane permeability. Eur J Biochem 228:1020–1029
Mark M, Walter R, Meredith DO, Reinhart WH (2001) Commercial taxane formulations induce stomatocytosis and increase blood viscosity. Br J Pharmacol 134:1207–1214
Loos WJ, Baker SD, Verweij J, Boonstra JG, Sparreboom A (2003) Clinical pharmacokinetics of unbound docetaxel: role of polysorbate 80 and serum proteins. Clin Pharmacol Ther 74:364–371
Baker SD, Li J, ten Tije AJ, Figg WD, Graveland W, Verweij J, Sparreboom A (2005) Relationship of systemic exposure to unbound docetaxel and neutropenia. Clin Pharmacol Ther 77:43–53
Engels FK, Mathot RA, Verweij J (2007) Alternative drug formulations of docetaxel: a review. Anticancer Drugs 18:95–103
Bracq E, Lahiani-Skiba M, Guerbet M (2008) Ethical observations on the choice of parenteral solvents. Choice of parenteral solvent. Drug Dev Ind Pharm 34:1306–1310
Hou W, Watters JW, McLeod HL (2004) Simple and rapid docetaxel assay in plasma by protein precipitation and high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 804:263–267
Brunsvig PF, Andersen A, Aamdal S, Kristensen V, Olsen H (2007) Pharmacokinetic analysis of two different docetaxel dose levels in patients with non-small cell lung cancer treated with docetaxel as monotherapy or with concurrent radiotherapy. BMC Cancer 7:197
Launay-Iliadis MC, Bruno R, Cosson V, Vergniol JC, Oulid-Aissa D, Marty M, Clavel M, Aapro M, Le Bail N, Iliadis A (1995) Population pharmacokinetics of docetaxel during phase I studies using nonlinear mixed-effect modeling and nonparametric maximum-likelihood estimation. Cancer Chemother Pharmacol 37:47–54
Rosing H, Lustig V, van Warmerdam LJ, Huizing MT, ten Bokkel Huinink WW, Schellens JH, Rodenhuis S, Bult A, Beijnen JH (2000) Pharmacokinetics and metabolism of docetaxel administered as a 1-h intravenous infusion. Cancer Chemother Pharmacol 45:213–218
Chow SC, Wang H (2001) On sample size calculation in bioequivalence trials. J Pharmacokinet Pharmacodyn 28:155–169
Schuirmann DJ (1987) A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm 15:657–680
Esmaeli B, Hortobagyi GN, Esteva FJ, Booser D, Ahmadi MA, Rivera E, Arbuckle R, Delpassand E, Guerra L, Valero V (2002) Canalicular stenosis secondary to weekly versus every-3-weeks docetaxel in patients with metastatic breast cancer. Ophthalmology 109:1188–1191
Ferraresi V, Milella M, Vaccaro A, D’Ottavio AM, Papaldo P, Nistico C, Thorel MF, Marsella A, Carpino A, Giannarelli D, Terzoli E, Cognetti F (2000) Toxicity and activity of docetaxel in anthracycline-pretreated breast cancer patients: a phase II study. Am J Clin Oncol 23:132–139
Valero V, Holmes FA, Walters RS, Theriault RL, Esparza L, Fraschini G, Fonseca GA, Bellet RE, Buzdar AU, Hortobagyi GN (1995) Phase II trial of docetaxel: a new, highly effective antineoplastic agent in the management of patients with anthracycline-resistant metastatic breast cancer. J Clin Oncol 13:2886–2894
Marchettini P, Stuart OA, Mohamed F, Yoo D, Sugarbaker PH (2002) Docetaxel: pharmacokinetics and tissue levels after intraperitoneal and intravenous administration in a rat model. Cancer Chemother Pharmacol 49:499–503
Zhao L, Wei YM, Zhong XD, Liang Y, Zhang XM, Li W, Li BB, Wang Y, Yu Y (2009) PK and tissue distribution of docetaxel in rabbits after i.v. administration of liposomal and injectable formulations. J Pharm Biomed Anal 49:989–996
Musumeci T, Ventura CA, Giannone I, Ruozi B, Montenegro L, Pignatello R, Puglisi G (2006) PLA/PLGA nanoparticles for sustained release of docetaxel. Int J Pharm 325:172–179
Gao K, Sun J, Liu K, Liu X, He Z (2008) Preparation and characterization of a submicron lipid emulsion of docetaxel: submicron lipid emulsion of docetaxel. Drug Dev Ind Pharm 34:1227–1237
Yang M, Ding Y, Zhang L, Qian X, Jiang X, Liu B (2007) Novel thermosensitive polymeric micelles for docetaxel delivery. J Biomed Mater Res A 81:847–857
Quaglia F, Ostacolo L, Mazzaglia A, Villari V, Zaccaria D, Sciortino MT (2009) The intracellular effects of non-ionic amphiphilic cyclodextrin nanoparticles in the delivery of anticancer drugs. Biomaterials 30:374–382
Liu Y, Li K, Pan J, Liu B, Feng SS (2010) Folic acid conjugated nanoparticles of mixed lipid monolayer shell and biodegradable polymer core for targeted delivery of Docetaxel. Biomaterials 31:330–338
Du W, Hong L, Yao T, Yang X, He Q, Yang B, Hu Y (2007) Synthesis and evaluation of water-soluble docetaxel prodrugs-docetaxel esters of malic acid. Bioorg Med Chem 15:6323–6330
Huang XX, Zhou CL, Wang H, Chen C, Yu SQ, Xu Q, Zhu YY, Ren Y (2011) Pharmacokinetics, efficacy, and safety evaluation of docetaxel/hydroxypropyl-sulfobutyl-beta-cyclodextrin inclusion complex. AAPS PharmSciTech 12:665–672
Acknowledgments
Chong Kun Dang Pharmaceutical Corporation provided financial support and study drugs to conduct this clinical study.
Conflict of interest
The authors have no vested interest of any kind in the materials or services referred to in this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Funded by Chong Kun Dang Pharmaceutical corp.; ClinicalTrials.gov number, NCT01019941.
Rights and permissions
About this article
Cite this article
Cho, E.K., Park, JY., Lee, K.H. et al. Open-label, randomized, single-dose, crossover study to evaluate the pharmacokinetics and safety differences between two docetaxel products, CKD-810 and Taxotere injection, in patients with advanced solid cancer. Cancer Chemother Pharmacol 73, 9–16 (2014). https://doi.org/10.1007/s00280-013-2264-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2264-0