Abstract
Purpose
Kahalalide F (KF) is a dehydroaminobutyric acid-containing peptide from marine origin with activity against several human malignant cell lines. This dose-escalating phase I clinical trial evaluated the maximum tolerated dose (MTD), and the recommended dose for further phase II studies (RD) of weekly KF given as a prolonged (3- to 24-h) intravenous (i.v.) infusion.
Methods
Eligible patients with advanced solid tumors and adequate performance status, hematologic, renal, and hepatic function were recruited into this study.
Results
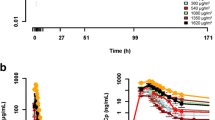
A total of 106 patients were treated with KF at four different weekly schedules: 3-h (n = 40), 24-h (n = 59), and two transitional schedules [6-h (n = 4) and 12-h (n = 3)]. For the 3-h weekly schedule, the MTD was 1,200 μg/m2 and the RD was 1,000 μg/m2. For the 24-h weekly schedule, the MTD was reached (6,650 μg/m2), but the RD could not be confirmed. Asymptomatic and reversible grade 3/4 transaminase increase was the most common dose-limiting toxicity in both schedules. Fatigue, paresthesia, pruritus, nausea, vomiting, and rash were the most common KF-related adverse events. No major deviations from linearity were detected in the pharmacokinetic (PK) profiles of both schedules, which showed a narrow distribution and short body residence. Prolonged disease stabilization (≥3 months) occurred in eight patients: two with the 3-h schedule and six with the 24-h schedule.
Conclusions
Administration of KF as prolonged weekly infusion appears feasible, with 3-h and 24-h infusion times having an acceptable safety profile.
Similar content being viewed by others
References
Hamann MT, Scheuer PJ (1993) Kahalalide F: a bioactive depsipeptide from the sacoglossan mollusk Elysia rufescens and the green alga Bryopsis sp. J Am Chem Soc 115:5825–5826
Hamann MT, Otto CS, Scheuer PJ, Dunbar DC (1996) Kahalalides: bioactive peptides from a marine mollusk elysia rufescens and its algal diet bryopsis sp. (1). J Org Chem 61:6594–6600
Lopez-Macia A, Jimenez JC, Royo M, Giralt E, Albericio F (2001) Synthesis and structure determination of kahalalide F (1,2). J Am Chem Soc 123:11398–11401
Hanauske AR, Hanauske U, Von Hoff DD (1985) The human tumor clonogenic assay in cancer research and therapy. Curr Probl Cancer 9:1–66
Suarez Y, Gonzalez L, Cuadrado A, Berciano M, Lafarga M, Munoz A (2003) Kahalalide F, a new marine-derived compound, induces oncosis in human prostate and breast cancer cells. Mol Cancer Ther 2:863–872
Garcia-Rocha M, Bonay P, Avila J (1996) The antitumoral compound Kahalalide F acts on cell lysosomes. Cancer Lett 99:43–50
Janmaat ML, Rodriguez JA, Jimeno J, Kruyt FA, Giaccone G (2005) Kahalalide F induces necrosis-like cell death that involves depletion of ErbB3 and inhibition of Akt signaling. Mol Pharmacol 68:502–510
Wosikowski K, Schuurhuis D, Johnson K, Paull KD, Myers TG, Weinstein JN, Bates SE (1997) Identification of epidermal growth factor receptor and c-erbB2 pathway inhibitors by correlation with gene expression patterns. J Natl Cancer Inst 89:1505–1515
Faircloth G, Cuevas C (2006) Kahalalide F and ES285: potent anticancer agents from marine molluscs. Prog Mol Subcell Biol 43:363–379
Rademaker-Lakhai JM, Horenblas S, Meinhardt W, Stokvis E, de Reijke TM, Jimeno JM, Lopez-Lazaro L, Lopez Martin JA, Beijnen JH, Schellens JH (2005) Phase I clinical and pharmacokinetic study of kahalalide F in patients with advanced androgen refractory prostate cancer. Clin Cancer Res 11:1854–1862
Pardo B, Paz-Ares L, Tabernero J, Ciruelos E, Garcia M, Salazar R, Lopez A, Blanco M, Nieto A, Jimeno J, Izquierdo MA, Trigo JM (2008) Phase I clinical and pharmacokinetic study of kahalalide F administered weekly as a 1-hour infusion to patients with advanced solid tumors. Clin Cancer Res 14:1116–1123
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Stokvis E, Rosing H, Lopez-Lazaro L, Rodriguez I, Jimeno JM, Supko JG, Schellens JH, Beijnen JH (2002) Quantitative analysis of the novel depsipeptide anticancer drug Kahalalide F in human plasma by high-performance liquid chromatography under basic conditions coupled to electrospray ionization tandem mass spectrometry. J Mass Spectrom 37:992–1000
Stokvis E, Rosing H, Lopez-Lazaro L, Schellens JH, Beijnen JH (2004) Switching from an analogous to a stable isotopically labeled internal standard for the LC-MS/MS quantitation of the novel anticancer drug Kahalalide F significantly improves assay performance. Biomed Chromatogr 18:400–402
Martin-Algarra S, Espinosa E, Rubio J, Lopez Lopez JJ, Manzano JL, Carrion LA, Plazaola A, Tanovic A, Paz-Ares L (2009) Phase II study of weekly Kahalalide F in patients with advanced malignant melanoma. Eur J Cancer 45:732–735
Gao J, Hamann MT (2011) Chemistry and biology of kahalalides. Chem Rev 111:3208–3235. doi:10.1021/cr100187n
Provencio M, Izquierdo A, Viñolas N, Paz-Ares L, Feliu J, Constenla M, De las Heras B, Erustes M, Izquierdo MA, Rossell R (2006) Phase II clinical trial of Kahalalide F (KF) as a second line therapy in patients (pts) with advanced non-small cell lung cancer (NSCLC). Ann Oncol 17:Abstract #755P
Jimeno J, Lopez-Martin JA, Ruiz-Casado A, Izquierdo MA, Scheuer PJ, Rinehart K (2004) Progress in the clinical development of new marine-derived anticancer compounds. Anticancer Drugs 15:321–329
Conflict of interest
Josep Tabernero has participated in advisory boards of Pharma Mar, and his institution has received research funding from Pharma Mar to conduct clinical trials. Cinthya Coronado, Arturo Soto Matos-Pita, Bernardo Miguel-Lillo, Martin Cullell-Young, and Jorge Luis Iglesias Dios are currently PharmaMar employees. All other authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salazar, R., Cortés-Funes, H., Casado, E. et al. Phase I study of weekly kahalalide F as prolonged infusion in patients with advanced solid tumors. Cancer Chemother Pharmacol 72, 75–83 (2013). https://doi.org/10.1007/s00280-013-2170-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2170-5