Abstract
Purpose
PM00104 (Zalypsis®) is a synthetic tetrahydroisoquinoline alkaloid with potent antiproliferative activity against tumor cell lines. This phase I study evaluated the safety, dose-limiting toxicities (DLTs), recommended dose for phase II trials (RD), pharmacokinetics (PK) and preliminary antitumor activity of PM00104 as a 24-h intravenous (i.v.) infusion every 3 weeks (q3wk).
Methods
Thirty-seven patients with refractory advanced solid tumors received PM00104 in a toxicity-guided dose escalation study design (3 + 3 patients per cohort). Plasma samples were collected for PK analysis.
Results
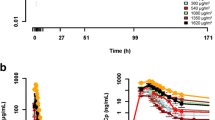
DLTs comprised severe neutropenia lasting >5 days (n = 4 patients), vomiting, thrombocytopenia, transaminase increases (n = 2 each), fatigue, tumor pain, myalgia, muscle stiffness, creatine phosphokinase increase and dosing delay >2 weeks due to moderate fatigue (n = 1 each). The RD was 4.0 mg/m2. Most PM00104-related adverse events at the RD were mild or moderate; the most common were nausea, vomiting and fatigue. Myelosuppression and transaminase increases were transient and manageable. PK parameters increased linearly with dose. Higher PM00104 PK exposure was related to a decrease in hemoglobin, neutrophils, platelets and white blood cells. Area under the curve was directly correlated with both incidence and severity of nausea and vomiting. Three patients with hepatocellular carcinoma, esophageal adenocarcinoma and prostate adenocarcinoma had response evaluation criteria in solid tumors stable disease ≥3 months.
Conclusions
PM00104 given as 24-h i.v. infusion q3wk has predictable and manageable toxicity, but resulted in more myelotoxicity (because of the higher dose level achieved as the RD) and a similar drug clearance compared to 1-h infusion schedules. Preliminary evidence of antitumor activity was observed.

Similar content being viewed by others
References
Ocio EM, Maiso P, Chen X, Garayoa M, Alvarez-Fernandez S, San-Segundo L, Vilanova D, Lopez-Corral L, Montero JC, Hernandez-Iglesias T, de Alava E, Galmarini C, Aviles P, Cuevas C, San-Miguel JF, Pandiella A (2009) Zalypsis: a novel marine-derived compound with potent antimyeloma activity that reveals high sensitivity of malignant plasma cells to DNA double-strand breaks. Blood 113:3781–3791
Leal JF, Garcia-Hernandez V, Moneo V, Domingo A, Bueren-Calabuig JA, Negri A, Gago F, Guillen-Navarro MJ, Aviles P, Cuevas C, Garcia-Fernandez LF, Galmarini CM (2009) Molecular pharmacology and antitumor activity of Zalypsis in several human cancer cell lines. Biochem Pharmacol 78:162–170
Elices M, LePage DJ, Grant WL, Sasak HT, Caylor T, Guillen MJ, Martin J, Cuevas C, Aviles P, Faircloth G (2005) Profile of Zalypsis (PM00104) against various human solid tumors. Clin Cancer Res 11:9085s–9086s
Elices M, Sasak H, Caylor T, Grant W, Guillen MJ, Martin J, Cuevas C, Aviles P, Faircloth G (2005) Antitumor activity of the novel investigational compound PM00104 (Zalypsis). AACR American Association for Cancer Research; 96th Annual Meeting: Abstract #5882
Elices M, Grant W, Harper C, Guillen MJ, Cuevas C, Faircloth G, Aviles P (2005) The novel compound PM00104 exhibits significant in vivo activity against breast tumors. AACR American Association for Cancer Research; 96th Annual Meeting: Abstract #623
Guirouilh-Barbat J, Antony S, Pommier Y (2009) Zalypsis (PM00104) is a potent inducer of gamma-H2AX foci and reveals the importance of the C ring of trabectedin for transcription-coupled repair inhibition. Mol Cancer Ther 8:2007–2014
Magro PG, Kolb A, Alvarez E, Mandola MV, Scotto KW (2007) Characterization of tumor cell response to the novel anti-tumor agent Zalypsis. American Association for Cancer Research (AACR) Meeting Abstracts: Abstract #1529
Yin J, Aviles P, Guillen MJ, Ly C, Lee W, Munt S, Cuevas C, Faircloth GT (2005) Pharmacokinetic evaluation of a novel anti-tumor agent, Zalypsis (PM00104), in mice and dogs. Clin Cancer Res 11:9072 s (Abstract B9163)
Yin J, Aviles P, McCarroll L, Wright T, Guillen MJ, Ly C, Lee W, Munt S, Cuevas C, Faircloth GT (2006) Toxicokinetic evaluation of a novel anti-tumor agent, Zalypsis(R) (PM00104), in dogs. AACR American Association for Cancer Research; 97th Annual Meeting: Abstract 3095
Simon R, Freidlin B, Rubinstein L, Arbuck SG, Collins J, Christian MC (1997) Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst 89:1138–1147
National Cancer Institute (2006) Common terminology criteria for adverse events v3.0 (CTCAE). http://www.eortc.be/services/doc/ctc/ctcaev3.pdf
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Yin J, Aviles P, Lee W, Ly C, Guillen MJ, Munt S, Cuevas C, Faircloth G (2005) Development of a liquid chromatography/tandem mass spectrometry assay for the quantification of PM00104, a novel antineoplastic agent, in mouse, rat, dog, and human plasma. Rapid Commun Mass Spectrom 19:689–695
Beal S, Sheiner L, Boeckmann A, Bauer R (2009) NONMEM User’s Guides. (1989–2009). Icon Development Solutions, Ellicott City, MD, USA
Yap TA, Cortes-Funes H, Shaw H, Rodriguez R, Olmos D, Lal R, Fong PC, Tan DS, Harris D, Capdevila J, Coronado C, Alfaro V, Soto-Matos A, Fernandez-Teruel C, Siguero M, Tabernero JM, Paz-Ares L, de Bono JS, Lopez-Martin JA (2012) First-in-man phase I trial of two schedules of the novel synthetic tetrahydroisoquinoline alkaloid PM00104 (Zalypsis) in patients with advanced solid tumours. Br J Cancer 106:1379–1385. doi:10.1038/bjc.2012.99
Massard C, Margetts J, Amellal N, Drew Y, Bahleda R, Stevens P, Armand JP, Calvert H, Soria JC, Coronado C, Kahatt C, Alfaro V, Siguero M, Fernandez-Teruel C, Plummer R (2012) Phase I study of PM00104 (Zalypsis(R)) administered as a 1-hour weekly infusion resting every fourth week in patients with advanced solid tumors. Investigational new drugs: Jun 12 (Epub ahead of print). doi:10.1007/s10637-012-9843-5
Kris MG, Hesketh PJ, Somerfield MR, Feyer P, Clark-Snow R, Koeller JM, Morrow GR, Chinnery LW, Chesney MJ, Gralla RJ, Grunberg SM (2006) American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol 24:2932–2947
Gonzalez-Sales M, Valenzuela B, Perez-Ruixo C, Fernandez Teruel C, Miguel-Lillo B, Soto-Matos A, Perez-Ruixo JJ (2012) Population pharmacokinetic-pharmacodynamic analysis of neutropenia in cancer patients receiving PM00104 (Zalypsis((R))). Clin Pharmacokinet 51:751–764. doi:10.1007/s40262-012-0011-z
Perez-Ruixo C, Valenzuela B, Fernandez Teruel C, Gonzalez-Sales M, Miguel-Lillo B, Soto-Matos A, Perez-Ruixo JJ (2012) Population pharmacokinetics of PM00104 (Zalypsis((R))) in cancer patients. Cancer Chemother Pharmacol 69:15–24
Acknowledgments
The authors would like to acknowledge Ms Moira Stewart and Ms Patricia Campbell from the Edinburgh Cancer Centre for their involvement in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Capdevila, J., Clive, S., Casado, E. et al. A phase I pharmacokinetic study of PM00104 (Zalypsis®) administered as a 24-h intravenous infusion every 3 weeks in patients with advanced solid tumors. Cancer Chemother Pharmacol 71, 1247–1254 (2013). https://doi.org/10.1007/s00280-013-2119-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2119-8