Abstract
Introduction
Malignant pleural effusion (MPE) is a frequent complication in many types of tumors diminishing the patient’s ability to perform activities. Despite various studies on talc treatment, some doubts about its safety and effectiveness remain, so the search for a more ideal intrapleural agent continues. We analyzed the effectiveness and safety of intrapleural paclitaxel in ovarian and breast cancer patients.
Patients and methods
The primary endpoint was overall response rate (ORR); secondary objectives included time to progression (TTP), overall survival (OS) and safety of intrapleural paclitaxel. Pharmacokinetics of the drug was also analyzed.
After drainage of pleural effusion and lung re-expansion, paclitaxel 120 mg/m2 diluted in normal saline was infused through a preinserted catheter which was immediately closed and reopened 24 h later. Blood and pleural fluid samples were collected 1, 4 and 24 h after the end of paclitaxel instillation. When MPE was less than 200 ml/24 h the catheter was removed. Chest radiographs were performed at the beginning of intrapleural paclitaxel, at 1 and 2 months later or with clinical deterioration.
Results
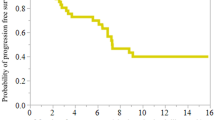
We enrolled 18 patients with recurrent MPE: 11 with ovarian cancer and 7 with breast cancer. ORR was 77.8% at 1 month and 88.8%. at 2 months. Median TTP was 5.5 months (CI 95% 0.9–10.1) and median OS was 8.9 months (CI 95% 0.1–17.6). Patients achieving a complete response obtained a statistically significant longer survival than did patients with partial response or progressive disease. Chest pain, fever, and dyspnea were the most frequent side effects. Intrapleural paclitaxel concentrations were very high (mean ± SD = 478 ± 187 mg/l) and declined slowly (mean 24 h reduction ~30%). Detectable but low taxol plasma levels were found in most patients (mean ± SD = 0.045 ± 0.073 mg/l).
Conclusion
Intrapleural paclitaxel is a safe and effective palliative treatment for MPE from breast and ovarian cancers and may be integrated with systemic chemotherapy.



Similar content being viewed by others
References
Cheng D, Rodriguez RM, Perkett EA, Rogers J, Bienvenu G, Lappalainen U, Light RW (1999) Vascular endothelial growth factor in pleural fluid. Chest 116:760–765
Bennett R, Maskell N (2005) Management of malignant pleural effusions. Curr Opin Pulm Med 11:296–300
Lombardi G, Zustovich F, Nicoletto MO, Donach M, Artioli G, Pastorelli D (2010) Diagnosis and treatment of malignant pleural effusion: a systematic literature review and new approaches. Am J Clin Oncol 33:420–423
Janssen JP, Collier G, Astoul P, Tassi GF, Noppen M, Rodriguez-Panadero F, Loddenkemper R, Herth FJ, Gasparini S, Marquette CH, Becke B, Froudarakis ME, Driesen P, Bolliger CT, Tschopp JM (2007) Safety of pleurodesis with talc poudrage in malignant pleural effusion: a prospective cohort study. Lancet 369:1535–1539
Heffner JE, Klein JS (2008) Recent advances in the diagnosis and management of malignant pleural effusions. Mayo Clin Proc 83:235–250
Perng RP, Chen YM, Wu MF, Chou KC, Lin WC, Liu JM, Whang-Peng J (1998) Phase II trial of intrapleural paclitaxel injection for non-small-cell lung cancer patients with malignant pleural effusions. Respir Med 92:473–479
Perng RP, Wu MF, Lin SY, Chen YM, Lin JY, Whang-Peng J (1997) A phase I feasibility and pharmacokinetic study of intrapleural paclitaxel in patients with malignant pleural effusions. Anticancer Drugs 8:565–573
van de Molengraft FJ, Vooijs GP (1989) Survival of patients with malignancy-associated effusions. Acta Cytol 33:911–916
Lynch TJ Jr (1993) Management of malignant pleural effusions. Chest 103:385S–389S
Shaw P, Agarwal R (2004) Pleurodesis for malignant pleural effusions. Cochrane Database Syst Rev (1):CD002916
Tohda Y, Iwanaga T, Takada M, Yana T, Kawahara M, Negoro S, Okishio K, Kudoh S, Fukuoka M, Furuse K (1999) Intrapleural administration of cisplatin and etoposide to treat malignant pleural effusions in patients with non-small cell lung cancer. Chemotherapy 45:197–204
Shoji T, Tanaka F, Yanagihara K, Inui K, Wada H (2002) Phase II study of repeated intrapleural chemotherapy using implantable access system for management of malignant pleural effusion. Chest 121:821–824
Lerza R, Vannozzi MO, Tolino G, Viale M, Bottino GB, Bogliolo G, Cerruti A, Castello G, Mencoboni M, Reggiardo G, Pannacciulli I, Esposito M (1997) Carboplatin and cisplatin pharmacokinetics after intrapleural combination treatment in patients with malignant pleural effusion. Ann Oncol 8:385–391
Ichinose Y, Tsuchiya R, Koike T, Yasumitsu T, Nakamura K, Tada H, Yoshimura H, Mitsudomi T, Nakagawa K, Yokoi K, Kato H (2002) A prematurely terminated phase III trial of intraoperative intrapleural hypotonic cisplatin treatment in patients with resected non-small cell lung cancer with positive pleural lavage cytology: the incidence of carcinomatous pleuritis after surgical intervention. J Thorac Cardiovasc Surg 123:695–699
Hausheer FH, Yarbro JW (1985) Diagnosis and treatment of malignant pleural effusion. Semin Oncol 12:54–75
Wang X, Zhou J, Wang Y, Zhu Z, Lu Y, Wei Y, Chen L (2010) A phase I clinical and pharmacokinetic study of paclitaxel liposome infused in non-small cell lung cancer patients with malignant pleural effusions. Eur J Cancer 46:1474–1480
Jones DR, Taylor MD, Petroni GR, Shu J, Burks SG, Daniel TM, Gillenwater HH (2010) Phase I trial of intrapleural docetaxel administered through an implantable catheter in subjects with a malignant pleural effusion. J Thorac Oncol 5:75–81
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lombardi, G., Nicoletto, M.O., Gusella, M. et al. Intrapleural paclitaxel for malignant pleural effusion from ovarian and breast cancer: a phase II study with pharmacokinetic analysis. Cancer Chemother Pharmacol 69, 781–787 (2012). https://doi.org/10.1007/s00280-011-1765-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-011-1765-y