Abstract
Purpose
The primary objective of this Phase I study was to assess the safety and tolerability of the vascular endothelial growth factor signalling inhibitor cediranib in combination with cisplatin plus an oral fluoropyrimidine, in Japanese patients with previously untreated advanced gastric cancer.
Methods
Patients received continuous, once-daily oral doses of cediranib 20 mg in combination with either cisplatin (60 mg/m2 iv day 1) plus S-1 (40–60 mg bid, days 1–21) every 5 weeks for a maximum of eight cycles [Arm A]; or cisplatin (80 mg/m2 iv, day 1) plus capecitabine (1,000 mg/m2 bid, days 1–14) every 3 weeks for a maximum of six cycles [Arm B]. In both arms, the assessment period for dose-limiting toxicities (DLTs) was the first 21 days of cycle 1.
Results
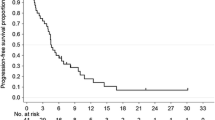
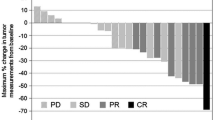
Fourteen patients (Arm A, n = 6; Arm B, n = 8) were enrolled and received at least one dose of cediranib. One patient in each arm experienced a DLT (Arm A; decreased appetite, grade 3; Arm B, decreased appetite, fatigue and hyponatraemia, all grade 3). Overall, the most common adverse events were decreased appetite, fatigue and nausea (all n = 13 [92.9%]). Preliminary efficacy evaluation showed one confirmed (Arm A) and three unconfirmed (Arm A, n = 1; Arm B, n = 2) partial responses that were ongoing at data cut-off.
Conclusions
Cediranib 20 mg/day in combination with cisplatin and S-1 or capecitabine was tolerable, with no new toxicities identified, and showed preliminary evidence of antitumour activity.


Similar content being viewed by others
References
GLOBOCAN statistics. 2008. Available at http://globocan.iarc.fr/
Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M (1993) Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer 72:37–41
Glimelius B, Hoffman K, Haglund U, Nyren O, Sjoden PO (1994) Initial or delayed chemotherapy with best supportive care in advanced gastric cancer. Ann Oncol 5:189–190
Pyrhonen S, Kuitunen T, Nyandoto P, Kouri M (1995) Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer 71:587–591
Fujii M, Kochi M, Takayama T (2010) Recent advances in chemotherapy for advanced gastric cancer in Japan. Surg Today 40:295–300
Ohtsu A, Shimada Y, Yoshida S, Saito H, Seki S, Morise K, Kurihara M (1994) Phase II study of protracted infusional 5-fluorouracil combined with cisplatinum for advanced gastric cancer: report from the Japan clinical oncology group (JCOG). Eur J Cancer 30A:2091–2093
Koizumi W, Kurihara M, Sasai T, Yoshida S, Morise K, Imamura A, Akazawa S, Betsuyaku T, Ohkubo S, Takahashi H et al (1993) A phase II study of combination therapy with 5′-deoxy-5-fluorouridine and cisplatin in the treatment of advanced gastric cancer with primary foci. Cancer 72:658–662
Koizumi W, Tanabe S, Saigenji K, Ohtsu A, Boku N, Nagashima F, Shirao K, Matsumura Y, Gotoh M (2003) Phase I/II study of S-1 combined with cisplatin in patients with advanced gastric cancer. Br J Cancer 89:2207–2212
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Takeuchi M (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9:215–221
Van Cutsem E, Kang Y, Chung H, Shen L, Sawaki A, Lordick F, Hill J, Lehle M, Feyereislova A, Bang Y (2009) Efficacy results from the ToGA trial: a phase III study of trastuzumab added to standard chemotherapy (CT) in first-line human epidermal growth factor receptor 2 (HER2)-positive advanced gastric cancer (GC). J Clin Oncol 27(18S):abst LBA4509
Ferrara N (2002) Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol 29:10–14
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342
Kabbinavar FF, Hambleton J, Mass RD, Hurwitz HI, Bergsland E, Sarkar S (2005) Combined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol 23:3706–3712
Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzen F, Cassidy J (2008) Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 26:2013–2019
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355:2542–2550
Heckman CA, Holopainen T, Wirzenius M, Keskitalo S, Jeltsch M, Wedge SR, Jürgensmeier JM (2007) Inhibition of VEGF-C-induced VEGFR-3 activity and lymphatic endothelial cell function by the tyrosine kinase inhibitor AZD2171. Proc Am Assoc Cancer Res 48:abst 2999
Wedge SR, Kendrew J, Hennequin LF, Valentine PJ, Barry ST, Brave SR, Smith NR, James NH, Dukes M, Curwen JO, Chester R, Jackson JA, Boffey SJ, Kilburn LL, Barnett S, Richmond GH, Wadsworth PF, Walker M, Bigley AL, Taylor ST, Cooper L, Beck S, Jürgensmeier JM, Ogilvie DJ (2005) AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res 65:4389–4400
Yamamoto N, Tamura T, Yamamoto N, Yamada K, Yamada Y, Nokihara H, Fujiwara Y, Takahashi T, Murakami H, Boku N, Yamazaki K, Puchalski TA, Shin E (2009) Phase I, dose escalation and pharmacokinetic study of cediranib (RECENTIN), a highly potent and selective VEGFR signaling inhibitor, in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol 64:1165–1172
Drevs J, Siegert P, Medinger M, Mross K, Strecker R, Zirrgiebel U, Harder J, Blum H, Robertson J, Jürgensmeier JM, Puchalski TA, Young H, Saunders O, Unger C (2007) Phase I clinical study of AZD2171, an oral vascular endothelial growth factor signaling inhibitor, in patients with advanced solid tumors. J Clin Oncol 25:3045–3054
Laurie SA, Gauthier I, Arnold A, Shepherd FA, Ellis PM, Chen E, Goss G, Powers J, Walsh W, Tu D, Robertson J, Puchalski TA, Seymour L (2008) Phase I and pharmacokinetic study of daily oral AZD2171, an inhibitor of vascular endothelial growth factor tyrosine kinases, in combination with carboplatin and paclitaxel in patients with advanced non-small-cell lung cancer: the National Cancer Institute of Canada clinical trials group. J Clin Oncol 26:1871–1878
Chen E, Jonker D, Gauthier I, Maclean M, Wells J, Powers J, Seymour L (2009) Phase I study of cediranib in combination with oxaliplatin and infusional 5-fluorouracil in patients with advanced colorectal cancer. Clin Cancer Res 15:1481–1486
Goss G, Shepherd FA, Laurie S, Gauthier I, Leighl N, Chen E, Feld R, Powers J, Seymour L (2009) A phase I and pharmacokinetic study of daily oral cediranib, an inhibitor of vascular endothelial growth factor tyrosine kinases, in combination with cisplatin and gemcitabine in patients with advanced non-small cell lung cancer: a study of the National Cancer Institute of Canada Clinical Trials Group. Eur J Cancer 45:782–788
LoRusso P, Shields AF, Gadgeel S, Vaishampayan U, Guthrie, T, Puchalski T, Xu J, Liu Q (2010) Cediranib in combination with various anticancer regimens: results of a phase I multi-cohort study. Invest New Drugs. doi:10.1007/s10637-010-9484-5. Epub ahead of print
Goss GD, Arnold A, Shepherd FA, Dediu M, Ciuleanu TE, Fenton D, Zukin M, Walde D, Laberge F, Vincent MD, Ellis PM, Laurie SA, Ding K, Frymire E, Gauthier I, Leighl NB, Ho C, Noble J, Lee CW, Seymour L (2010) Randomized, double-blind trial of carboplatin and paclitaxel with either daily oral cediranib or placebo in advanced non-small-cell lung cancer: NCIC clinical trials group BR24 study. J Clin Oncol 28:49–55
Robertson JD, Botwood NA, Rothenberg ML, Schmoll H-J (2009) Phase III trial of FOLFOX plus bevacizumab or cediranib (AZD2171) as first-line treatment of patients with metastatic colorectal cancer: HORIZON III. Clin Colorectal Cancer 8:59–60
AstraZeneca (2011) Global policy: bioethics. Available at http://www.astrazeneca.com/Responsibility/Code-policies-standards/Policiesstandards
Therasse P (2002) Measuring the clinical response. What does it mean? Eur J Cancer 38:1817–1823
vanCruijsen H, Voest EE, van Herpen CML, Hoekman K, Witteveen PO, Tjin-A-Ton ML, Punt CJ, Puchalski T, Milenkova T, Giaccone G (2006) Phase I evaluation of AZD2171, a highly potent, selective VEGFR signaling inhibitor, in combination with gefitinib, in patients with advanced tumors. J Clin Oncol 24(S18):abst 3017
Lee SS, Lee JL, Ryu MH, Chang HM, Kim TW, Kang HJ, Kim WK, Lee JS, Kang YK (2007) Combination chemotherapy with capecitabine (X) and Cisplatin (P) as first line treatment in advanced gastric cancer: experience of 223 patients with prognostic factor analysis. Jpn J Clin Oncol 37:30–37
Lee JO, Lee KW, Oh DY, Kim JH, Im SA, Kim TY, Bang YJ (2009) Combination chemotherapy with capecitabine and cisplatin for patients with metastatic hepatocellular carcinoma. Ann Oncol 20:1402–1407
Schulz-Utermoehl T, Spear M, Pollard CR, Pattison C, Rollison H, Sarda S, Ward M, Bushby N, Jordan A, Harrison M (2010) In vitro hepatic metabolism of cediranib, a potent vascular endothelial growth factor tyrosine kinase inhibitor: interspecies comparison and human enzymology. Drug Metab Dispos 38:1688–1697
Kim C, Lee J-L, Choi YH, Kang BW, Ryu M-H, Chang HM, Kim TW, Kang Y-K (2011) Phase I dose-finding study of sorafenib in combination with capecitabine and cisplatin as a first-line treatment in patients with advanced gastric cancer. Invest New Drugs. doi:10.1007/s10637-010-9531-2. Epub ahead of print
Kang Y, Ohtsu A, Van Cutsem E, Rha SY, Sawaki A, Park P, Lim H, Wu J, Langer B, Shah MA (2010) AVAGAST: a randomized, double-blind, placebo-controlled, phase III study of first-line capecitabine and cisplatin plus bevacizumab or placebo in patients with advanced gastric cancer (AGC). J Clin Oncol 28(18S):abst LBA4007
Acknowledgments
Funding for this study was provided by AstraZeneca. We thank Paul Williams, PhD, from Mudskipper Bioscience, who provided medical writing assistance funded by AstraZeneca.
Conflict of interest
X.S. and K.H.B. are employees of AstraZeneca and own stock. T.S., Y.Y, K.M., H.H., Y.S., D.T., K.T., T.E.N. and N.B. declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Satoh, T., Yamada, Y., Muro, K. et al. Phase I study of cediranib in combination with cisplatin plus fluoropyrimidine (S-1 or capecitabine) in Japanese patients with previously untreated advanced gastric cancer. Cancer Chemother Pharmacol 69, 439–446 (2012). https://doi.org/10.1007/s00280-011-1723-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-011-1723-8